To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Adiabatic invariantAn adiabatic invariant is a property of a physical system which stays constant when changes are made slowly. In thermodynamics, an adiabatic process is a change that occurs without heat flow and slowly compared to the time to reach equilibrium. In an adiabatic process, the system is in equilibrium at all stages. Under these conditions the entropy is constant. In mechanics, an adiabatic change is a slow deformation of the Hamiltonian where the fractional rate of change of the energy is much slower than the orbital frequency. The area enclosed by the different motions in phase space are then the adiabatic invariants. In quantum mechanics, an adiabatic change is one that occurs at a rate much slower than the difference in frequency between energy eigenstates. In this case, the energy states of the system do not make transitions, so that the quantum number is an adiabatic invariant. The old quantum theory was formulated by equating the quantum number of a system with its classical adiabatic invariant. This determined the form of the Bohr-Sommerfeld quantization rule: the quantum number is the area in phase space of the classical orbit. Additional recommended knowledge
ThermodynamicsIn thermodynamics, adiabatic changes are those which do not increase the entropy. They occur slowly, and allow heat flow only between objects at the same temperature. For isolated systems, an adiabatic change allows no heat to flow in or out. Adiabatic Expansion of an Ideal GasIf a container with an ideal gas is expanded instantaneously, the temperature of the gas doesn't change at all, because none of the molecules slow down. The molecules keep their kinetic energy, but now the gas occupies a bigger volume. But if the container is expanded slowly, so that the ideal gas pressure law holds at any time, the gas molecules lose energy at the rate that they do work on the expanding wall. The amount of work they do is the pressure times the area of the wall times the outward dispacement, which is the pressure times the change in the volume of the gas: If no heat enters the gas, the energy in the gas molecules is decreasing by the same amount. By definition, a gas is ideal when its temperature is only a function of the internal energy per particle, not the volume. So Where Cv is the specific heat at constant volume. When the change in energy is entirely due to work done on the wall, the change in temperature is given by: This gives a differential relationship between the changes in temperature and volume which can be integrated to find the invariant. The constant kB is just a unit conversion factor, which can be set equal to one: An expression for the adiabatic invariant is although any function of S is also invariant. This form is preferred because it is the entropy. In a molecular interpretation, it is the logarithm of the phase space volume of all gas states with energy E(T) and volume V. This is the available volume in phase space at energies E, as can be seen by writing down the energy. The different internal motions of the gas with total energy E define a sphere in 3N dimensions, with radius identifying T = E3 / 2 gives the thermodynamic form. The specific heat is 3/2, or 3 / 2kB in units where Wien's Law— Adiabatic Expansion of A Box of LightFor a box of radiation, ignoring quantum mechanics, the energy of a classical field in thermal equilibrium is infinite, since equipartition demands that each field mode has an equal energy on average and there are infinitely many modes. This is physically ridiculous, since it means that all energy will leak into high frequency electromagnetic waves over time. Still, without quantum mechanics, there are some things that can be said about the equilibrium distribution from thermodynamics alone, because there is still a notion of adiabatic invariance which relates boxes of different size. When a box is slowly expanded, the frequency of the light recoiling from the wall can be computed from the Doppler shift. If the wall is not moving, the light recoils at the same frequency. If the wall is moving slowly, the recoil frequency is only equal in the frame where the wall is stationary. In the frame where the wall is moving away from the light, the light coming in is bluer than the light coming out by twice the Doppler shift factor v/c. On the other hand, the energy in the light is also decreased when the wall is moving away, because the light is doing work on the wall by radiation pressure. Because the light is reflected, the pressure is equal to the twice the momentum carried by light, which it is E/c. The rate at which the pressure does work on the wall is found by multiplying by the velocity: This means that the change in frequency of the light is equal to the work done on the wall by the radiation pressure. The light which is reflected is changed both in frequency and in energy by the same amount: Since moving the wall slowly should keep a thermal distribution fixed, the probability that the light has energy E at frequency f must only be a function of E/f. This function cannot be determined from thermodynamic reasoning alone, and Wien made a guess for the form which was valid at high frequency. He supposed that the average energy in high frequency modes was suppressed by a Boltzmann-like factor. This is not the expected classical energy in the mode, which is 1 / 2β by equipartition, but a new and unjustified assumption which fit the high-frequency data. When the expectation value is added over all modes in a cavity, this is Wien's distribution, and it describes the thermodynamic distribution of energy in a classical gas of photons. The Wein law implicity assumes that light is statistically composed of packets that change energy and frequency in the same way. The entropy of a Wein gas scales as the volume to the power N, where N is the number of packets. This led Einstein to suggest that light is composed of localizable particles with energy propotional to the frequency. Then the entropy of the Wien gas can be given a statistical interpretation as the number of possible positions that the photons can be in. Classical Mechanics— Action VariablesSuppose that a Hamiltonian is slowly time varying, for example, a one dimensional harmonic oscillator with a changing frequency. The action J of a classical orbit is the area enclosed by the orbit in phase space. Since J is an integral over a full period, it is only a function of the energy. When the Hamiltonian is constant in time and J is constant in time, the canonically conjugate variable θ increases in time at a steady rate. So the constant H' can be used to change time derivatives along the orbit to partial derivatives with respect to θ at constant J. Differentiating the integral for J with respect to J gives an identity which fixes H': The integrand is the Poisson bracket of x and p. The Poisson bracket of two canonically conjugate quantities like x and p is equal to 1 in any canonical coordinate system. So
and H' is the inverse period. The variable θ increases by an equal amount in each period for all values of J— it is an angle-variable.
The Hamiltonian is a function of J only, and in the simple case of the Harmonic oscillator. When H has no time dependence, J is constant. When H is slowly time varying, the rate of change of J can be computed by reexpressing the integral for J The time derivative of this quantity is Replacing time derivatives with theta derivatives, So as long as the coordinates J,θ do not change appreciably over one period, this expression can be integrated by parts to give zero. This means that for slow variations, there is no lowest order change in the area enclosed by the orbit. This is the adiabatic invariance theorem— the action variables are adiabatic invariants. For a harmonic oscillator, the area in phase space of an orbit at energy E is the area of the ellipse of constant energy, The x-radius of this ellipse is Old Quantum TheoryAfter Planck identified that Wien's law can be extended to all frequencies, even very low ones, by interpolating with the classical equipartition law for radiation, physicists wanted to understand the quantum behavior of other systems. The Planck radiation law quantized the motion of the field oscillators in units of energy proportional to the frequency: This is the only sensible quantization. The quantum can only depend on the energy/frequency by adiabatic invariance, and since the energy must be additive when putting boxes end to end, the levels must be equally spaced. Einstein, followed by Debye, extended the domain of quantum mechanics by considering the sound modes in a solid as quantized oscillators. This model explained why the specific heat of solids approached zero at low temperatures, instead of staying fixed at 3kB as predicted by classical equipartition. At the Solvay conference, the question of quantizing other motions was raised, and Lorentz pointed out a problem. If you consider a quantum pendulum whose string is shortened very slowly, the quantum number of the pendulum cannot change because at no point is there a high enough frequency to cause a transition between the states. But the frequency of the pendulum changes when the string is shorter, so the quantum states change energy. Einstein responded that for slow pulling, the frequency and energy of the pendulum both change but the ratio stays fixed. This is analogous to Wien's observation that under slow motion of the wall the energy to frequency ratio of reflected waves is constant. The conclusion was that the quantities to quantize must be adiabatic invariants. This line of argument was extended by Sommerfeld into a general theory: the quantum numbers of an arbitrary mechanical system is given by the adiabatic action variable. Since the action variable in the harmonic oscillator is an integer, the general condition is: This condition was the foundation of the old quantum theory, which was able to predict the qualitative behavior of atomic systems. The theory is inexact for small quantum numbers, since it mixes classical and quantum concepts. But it was a useful half-way step to the new quantum theory. Plasma PhysicsIn plasma physics there are three adiabatic invariants of charged particle motion. The first adiabatic invariant, μThe magnetic moment of a gyrating particle,
is a constant of the motion (as long as q/m does not change). In fact, it is invariant to all orders in an expansion in ω / ωc, so the magnetic moment remains nearly constant even for changes at rates approaching the gyrofrequency. There are some important situations in which the magnetic moment is not invariant:
The second adiabatic invariant, JThe longitudinal invariant of a particle trapped in a magnetic mirror,
where the integral is between the two turning points, is also an adiabatic invariant. This guarantees, for example, that a particle in the ionosphere moving around the Earth will always return to the same line of force. The adiabatic condition is violated in transit-time magnetic pumping, where the length of a magnetic mirror is oscillated at the bounce frequency, resulting in net heating. The third adiabatic invariant, ΦThe total magnetic flux Φ enclosed by a drift surface is the third adiabatic invariant, associated with the periodic motion of mirror-trapped particles drifting around the axis of the system. Because this drift motion is relatively slow, Φ is often not conserved in practical applications. References
Categories: Thermodynamics | Plasma physics |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Adiabatic_invariant". A list of authors is available in Wikipedia. |





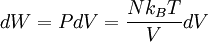
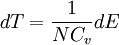
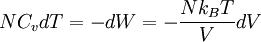
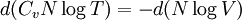
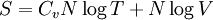
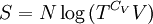
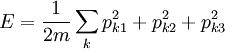
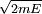 . Whose surface is a 3N-1 dimensional and the number of states scales as
. Whose surface is a 3N-1 dimensional and the number of states scales as 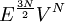
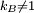 .
.
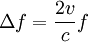
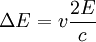
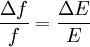
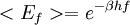
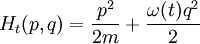
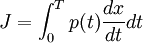
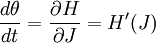
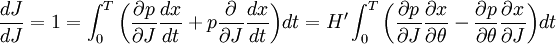
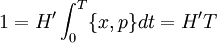
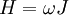
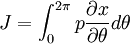
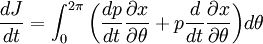
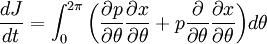
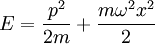
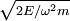 , while the p-radius of the ellipse is
, while the p-radius of the ellipse is 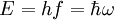
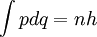
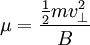 ,
,
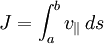 ,
,


