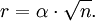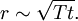To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Atomic diffusionAtomic diffusion is a process whereby the random thermally-activated hopping of atoms in a solid results in the net transport of atoms. For example, helium atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air molecules (e.g. oxygen, nitrogen) have lower mobilities and thus diffuse more slowly through the balloon wall. There is a concentration gradient in the balloon wall, because the balloon was initially filled with helium, and thus there is plenty of helium on the inside, but there is relatively little helium on the outside (helium is not a major component of air). The rate of transport is governed by the diffusivity and the concentration gradient. Additional recommended knowledgeIn crystalsIn the crystal solid state, the occurrence of diffusion is contingent upon the availability of point vacancies throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about. Since the prevalence of point vacancies increases in accordance with the Arrhenius equation, the rate of crystal solid state diffusion increases with temperature. For a single atom in a defect-free crystal, the movement can be described by the "random walk" model. In this 3-dimensional picture,missing picture it can be shown that after n jumps of length α the atom will have moved, on average, a distance of: If the jump frequency is given by T (in jumps per second) and time is given by t, then r is proportional to the square root of Tt: See also
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Atomic_diffusion". A list of authors is available in Wikipedia. |