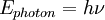To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Atomic emission spectrumThe atomic emission spectrum of an element is the set of frequencies of the electromagnetic waves emitted by atoms of that element. Each atom's atomic emission spectrum is unique and can be used to determine if that element is part of an unknown compound. Additional recommended knowledgeThe emission spectrum characteristics of some elements are plainly visible to the naked eye when these elements are heated. For example, when platinum wire is dipped into a strontium nitrate solution and then inserted into a flame, the strontium atoms emit a red color. Similarly, when copper is inserted into a flame, the flame becomes light blue. These definite characteristics allow elements to be identified by their atomic emission spectrum. Not all lights emitted by the spectrum are viewable to the naked eye, it also includes ultra violet rays and infra red lighting. The fact that only certain colors appear in an element's atomic emission spectrum means that only certain frequencies of light are emitted. Each of these frequencies are related to energy by the formula: herein E is energy, h is Planck's constant and ν is the frequency. This concludes that only photons having certain energies are emitted by the atom. The principle of the atomic emission spectrum explains the varied colors in neon signs, as well as chemical flame test results mentioned above. The frequencies of light that an atom can emit are dependent on states the electrons can be in. When excited, an electron moves to a higher energy level/orbital. When the electron falls back to its ground level the light is emitted. An emission spectrum is always the inverse of its absorption spectrum.
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Atomic_emission_spectrum". A list of authors is available in Wikipedia. |