To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Bond dipole momentThe bond dipole moment is a measure for the polarity of a chemical bond within a molecule. The bond dipole μ is given by:
Additional recommended knowledgeThe bond dipole is modeled as, +δ — δ- with a distance d between the partial charges +δ and δ-. It is a vector, pointing from minus to plus,[1] that is parallel to the bond. Chemists generally measure electrical dipole moments in debyes, represented by the symbol D. The SI unit for dipole moment is the coulomb-meter (1 C m = 2.9979 1029 D), δ is the amount of charge in coulombs, and d is in meters. For a complete molecule the total molecular dipole moment may be approximated as the vector sum of individual bond dipole moments. Often bond dipoles are obtained by the reverse process: a known total dipole of a molecule can be decomposed into bond dipoles. The reason for doing this is the transfer of bond dipole moments to molecules that have the same bonds, but for which the total dipole moment is not yet known. The vector sum of the transferred bond dipoles gives an estimate for the total (unknown) dipole of the molecule. The Bond Dipole is two atoms in a bond, such that the electronegativity of one atom changes and draws the electrons towards the other, causing a partial negative charge. There is an increase difference in polarity, and an increase in dipole. See alsoNote
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Bond_dipole_moment". A list of authors is available in Wikipedia. |


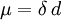 .
.





