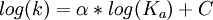To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Brønsted catalysis equationThe Brønsted catalysis equation or law or correlation, after Johannes Nicolaus Brønsted, gives the relationship between acid strength and catalytic activity. Additional recommended knowledge
Specific and general catalysis is also found in base catalysed reactions and base Brønsted equation also exists with constant β. The Brønsted equation gives information about a reaction mechanism. Reactions that have low values for proportionality constants α or β are considered to have a transition state closely resembling the reactant with little proton transfer. With a high value, proton transfer in the transition state is almost complete. In a study of a group of phenalene compounds it was concluded from Brønsted analysis that phenalene acidity is very different from either indene acidity or phenylene acidity 1.
References
Categories: Catalysts | Chemical kinetics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Brønsted_catalysis_equation". A list of authors is available in Wikipedia. |