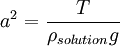To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Bubble rafts
Additional recommended knowledgeA material's observable and measurable mechanical properties strongly depend on its atomic and microstructural configuration and characteristics. This fact is intentionally ignored in Continuum Mechanics, which assumes a material to have no underlying microstructure and be uniform and semi-infinite throughout. Bubble Rafts take on the issue of microstructural and atomic length-scale behavior by modeling the {111} plane of a close-packed crystal. They accomplish this feat by assembling bubbles on a water surface, often with the help of amphiphilic soaps. These assembled bubbles act like atoms, diffusing, slipping, ripening, straining, and otherwise deforming in a way that models the behavior of the {111} plane of a close-packed crystal. The ideal (lowest energy) state of the assembly would undoubtedly be a perfectly regular single crystal, but just as in metals, the bubbles often form defects, grain boundaries, and multiple crystals. History of Bubble RaftsThe concept of Bubble Raft modelling was first presented in 1947 by Nobel Laureate Sir William Lawrence Bragg and J.F. Nye of Cambridge University's Cavendish Laboratory in the Proceedings of the Royal Society A[1]. Legend claims that Bragg conceived of bubble raft models while pouring oil into his lawn mower. He noticed that bubbles on the surface of the oil assembled into rafts resembling the {111} plane of close-packed crystals[2]. Nye and Bragg later presented a method of generating and controlling bubbles on the surface of a glycerine-water-oleic acid-triethanolamine solution, in assemblies of 100,000 or more sub-millimeter sized bubbles. In their paper [1], they go on at length about the microstructural phenomena observed in bubble rafts and hypothesized in metals. Relation to Crystal LatticesIn deforming a crystal lattice, one changes the energy and the interatomic potential felt by the atoms of the lattice. This interatomic potential is popularly (and mostly qualitatively) modeled using the Lennard-Jones potential, which consists of a balance between attractive and repulsive forces between atoms. The "atoms" in Bubble Rafts also exhibit such attractive and repulsive forces:
The portion of the equation to the left of the plus sign is the attractive force, and the portion to the right represents the repulsive force. U(ρ) is the interbubble potential R is the average bubble radius ρsolution is the density of the solution from which the bubbles are formed g is the gravitational constant ρ is the ratio of the distance between bubbles to the bubble radius Β is the radius of ring contact α is the ratio R/a of the bubble radius to the Laplace constant a, where
T is the surface tension A is a constant dependent upon the boundary conditions of the calculation K0 is a zeroth order modified Bessel function of the second kind[2] References[1] Bragg, L. & Nye, J.F., A Dynamical Model of a Crystal Structure, Proc. R. Soc. Lond. A, 190 (1023), 474-481 [1] [2] Laboratory Handout in MIT's 3.032: Mechanical Properties of Materials. [2] |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Bubble_rafts". A list of authors is available in Wikipedia. |





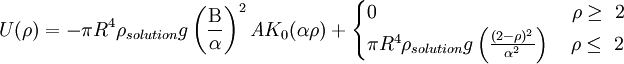 [2]
[2]