To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
CarbeneIn chemistry, a carbene is a highly reactive organic molecule with a divalent carbon atom with only six valence electrons and the general formula: R1R2C: (two substituents and two electrons).[1] The carbene comes in two varieties: a singlet and triplet. The singlet type has its carbon atom sp2 hybridised with an empty p-orbital extending above and below a plane containing R1 and R2 and the free electron pair. Typically these molecules are very short lived, although persistent carbenes are now known. The parent carbene is H2C: also called methylene. An often encountered carbene is Cl2C: or dichlorocarbene which can be generated in situ from chloroform and a strong base. Additional recommended knowledge
StructureGenerally there are two types of carbenes; singlet or triplet carbenes. Singlet carbenes have a pair of electrons and a sp2 hybrid structure. Triplet carbenes have two unpaired electrons. They may be either sp2 hybrid or linear sp hybrid. Most carbenes have a nonlinear triplet ground state with the exception of carbenes with nitrogen, oxygen, sulfur atoms, and dihalocarbenes. Singlet and triplet carbenes are named so because of the electronic spins they possess. Triplet carbenes are paramagnetic and may be observed by electron spin resonance spectroscopy if they can exist long enough without undergoing further reactions. The total spin of singlet carbenes is zero while that of triplet carbenes is one (in units of For simple hydrocarbons, triplet carbenes usually have energies 8 kcal/mol (33 kJ/mol) lower than singlet carbenes (see also Hund's rule of Maximum Multiplicity), thus, in general, triplet is the more stable state (the ground state) and singlet is the excited state species. Substituents that can donate electron pairs may stabilize the singlet state by delocalizing the pair into a empty p-orbital. If the energy of the singlet state is sufficiently reduced it will actually become the ground state. No viable strategies exist for triplet stabilization. The carbene called 9-fluorenylidene has been shown to be a rapidly equilibrating mixture of singlet and triplet states with an approximately 1.1 kcal/mol (4.6 kJ/mol) energy difference.[2]. It is however debatable whether diaryl carbenes such as the fluorene carbene are true carbenes because the electrons can delocalize to such an extent that they become in fact biradicals. In silico experiments suggest that triplet carbenes can be stabilized with electropositive groups such as trifluorosilyl groups [3]. ReactivitySinglet and triplet carbenes do not demonstrate the same reactivity. Singlet carbenes generally participate in cheletropic reactions as either electrophiles or nucleophiles. Singlet carbene with its unfilled p-orbital should be electrophilic. Triplet carbenes should be considered to be diradicals, and participate in stepwise radical additions. Triplet carbenes have to go through an intermediate with two unpaired electrons whereas singlet carbene can react in a single concerted step. Addition of singlet carbenes to olefinic double bonds is more stereoselective than that of triplet carbenes. Addition reactions with alkenes can be used to determine whether the singlet or triplet carbene is involved. Reactions of singlet methylene are stereospecific while those of triplet methylene are not. For instance the reaction of methylene generated from photolysis of diazomethane with cis-2-butene and trans-2-butene is stereospecific which proves that in this reaction methylene is a singlet.[4] Reactivity of a particular carbene depends on the substituent groups, preparation method, reaction conditions such as presence or absence of metals. Some of the reactions carbenes can do are insertions into C-H bonds, skeletal rearrangements, and additions to double bonds. Carbenes can be classified as nucleophilic, electrophilic, or ambiphilic. Reactivity is especially strongly influenced by substituents. For example, if a substituent is able to donate a pair of electrons, most likely carbene will not be electrophilic. Alkyl carbenes insert much more selectively than methylene, which does not differentiate between primary, secondary, and tertiary C-H bonds. Carbenes add to double bonds to form cyclopropanes. A concerted mechanism is available for singlet carbenes. Triplet carbenes do not retain stereochemistry in the product molecule. Addition reactions are commonly very fast and exothermic. The slow step in most instances is generation of carbene. A well-known reagent employed for alkene-to-cyclopropane reactions is Simmons-Smith reagent. This reagents is a system of copper, zinc, and iodine, where the active reagent is believed to be iodomethylzinc iodide. Reagent is complexed by hydroxy groups such that addition commonly happens syn to such group. Insertions are another common type of carbene reactions. The carbene basically interposes itself into an existing bond. The order of preference is commonly: 1. X-H bonds where X is not carbon 2. C-H bond 3. C-C bond. Insertions may or may not occur in single step. Intramolecular insertion reactions present new synthetic solutions. Generally, rigid structures favor such insertions to happen. When an intramolecular insertion is possible, no intermolecular insertions are seen. In flexible structures, five-membered ring formation is preferred to six-membered ring formation. Both inter- and intramolecular insertions are amendable to asymmetric induction by choosing chiral ligands on metal centers. Alkylidene carbenes are alluring in that they offer formation of cyclopentene moieties. To generate an alkylidene carbene a ketone can be exposed to trimethylsilyl diazomethane. Carbenes and carbene ligands in organometallic chemistryCarbenes can be stabilized as organometallic species. These transition metal carbene complexes fall into three categories, with the first two being the most clearly defined:
Generation of Carbenes
See alsoTransition metal carbene complex also known as carbenoids References
Categories: Reactive intermediates | Functional groups |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Carbene". A list of authors is available in Wikipedia. |





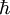 ). Bond angles are 125-140° for triplet methylene and 102° for singlet methylene (determined by EPR). Triplet carbenes are generally stable in gaseous state while singlet carbenes are often found in aqueous media.
). Bond angles are 125-140° for triplet methylene and 102° for singlet methylene (determined by EPR). Triplet carbenes are generally stable in gaseous state while singlet carbenes are often found in aqueous media.


