To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Carbonic acid
Carbonic acid (ancient name acid of air or aerial acid) has the formula H2CO3. It is also a name sometimes given to solutions of carbon dioxide in water, which contain small amounts of H2CO3. The salts of carbonic acids are called bicarbonates (or hydrogencarbonates) and carbonates. It is a weak acid. Carbonic acid should not be confused with carbolic acid, an antiquated name for phenol. Carbon dioxide dissolved in water is in equilibrium with carbonic acid:
The equilibrium constant at 25°C is Kh= 1.70×10−3: hence, the majority of the carbon dioxide is not converted into carbonic acid and stays as CO2 molecules. In the absence of a catalyst, the equilibrium is reached quite slowly. The rate constants are 0.039 s−1 for the forward reaction (CO2 + H2O → H2CO3) and 23 s−1 for the reverse reaction (H2CO3 → CO2 + H2O). Additional recommended knowledge
Role of carbonic acid in bloodCarbonic acid plays a very important role in mammalian blood. When CO2 enters the blood from various cells, it is combined with water to produce carbonic acid. It then has a H+ taken away from it to become bicarbonate (HCO3-). In order to transport the bicarbonate that is in the blood stream out of the body, it enters another red blood cell, has H+ attached to it to form carbonic acid once again, then has H2O taken away from it and is expelled from the red blood cell as CO2. Then the carbon dioxide is permitted to be expelled out of capillaries and into the lungs. The equilibrium between carbon dioxide and carbonic acid is very important for controlling the acidity of body fluids, and almost all living organisms have an enzyme, carbonic anhydrase, which catalyzes the conversion between the two compounds, increasing the reaction rate by a factor of nearly a billion. Acidity of carbonic acidCarbonic Acid is diprotic, that is it has two hydrogens which disassociate and thus two dissociation constants:
Care must be taken when quoting and using the first dissociation constant of carbonic acid. The value quoted above is correct for the H2CO3 molecule, and shows that it is a stronger acid than acetic acid or formic acid: this might be expected from the influence of the electronegative oxygen substituent. However, carbonic acid only ever exists in solution in equilibrium with carbon dioxide, and so the concentration of H2CO3 is much lower than the concentration of CO2, reducing the measured acidity. The equation may be rewritten as follows (c.f. sulfurous acid):
This figure is quoted as the dissociation constant of carbonic acid, although this is ambiguous: it might better be referred to as the acidity constant of carbon dioxide, as it is particularly useful for calculating the pH of CO2 solutions. pH and composition of a carbonic acid solutionAt a given temperature, the composition of a pure carbonic acid solution (or of a pure CO2 solution) is completely determined by the partial pressure
The corresponding equilibrium equations together with the
Remark: As noted above, [CO32−] may be neglected for this specific problem, resulting in the following very precise analytical expression for [H+]: Instability of carbonic acidIt has long been recognized that it is impossible to obtain pure hydrogen bicarbonate at room temperatures (about 20 °C or about 70 °F). However, in 1991 scientists at NASA's Goddard Space Flight Center (USA) succeeded in making the first pure H2CO3 samples. They did so by exposing a frozen mixture of water and carbon dioxide to high-energy radiation, and then warming to remove the excess water. The carbonic acid that remained was characterized by infrared spectroscopy. The fact that the carbonic acid was prepared by irradiating a solid H2O + CO2 mixture has given rise to suggestions that H2CO3 might be found in outer space, where frozen ices of H2O and CO2 are common, as are cosmic rays and ultraviolet light, to help them react. The same carbonic acid polymorph (denoted beta-carbonic acid) was prepared by a cryotechnique at the University of Innsbruck: alternating layers of glassy aqueous solutions of bicarbonate and acid were heated in vacuo, which causes protonation of bicarbonate, and the solvent was subsequently removed. A second polymorph (denoted alpha-carbonic acid) was prepared by the same technique at the University of Innsbruck using methanol rather than water as a solvent. It has since been shown, by theoretical calculations, that the presence of even a single molecule of water causes carbonic acid to revert to carbon dioxide and water fairly quickly. Pure carbonic acid is predicted to be stable in the gas phase, in the absence of water, with a calculated half-life of 180,000 years. There is a hypothetical acid orthocarbonic acid which is even more hydrated, being H4CO4. References
See also
Categories: Acids | Inorganic carbon compounds | Carbonates |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Carbonic_acid". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





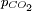 of carbon dioxide above the solution. To calculate this composition, account must be taken of the above equilibria between the three different carbonate forms (H2CO3, HCO3− and CO32−) as well as of the hydratation equilibrium between dissolved CO2 and H2CO3 with constant
of carbon dioxide above the solution. To calculate this composition, account must be taken of the above equilibria between the three different carbonate forms (H2CO3, HCO3− and CO32−) as well as of the hydratation equilibrium between dissolved CO2 and H2CO3 with constant ![\scriptstyle K_h=\frac{[H_2CO_3]}{[CO_2]}](images/math/5/4/8/548164e9dafca2231c39541c7e511fa1.png) (see above) and of the following equilibrium between the dissolved CO2 and the gaseous CO2 above the solution:
(see above) and of the following equilibrium between the dissolved CO2 and the gaseous CO2 above the solution:
![\scriptstyle \frac{[CO_2]}{p_{CO_2}}=\frac{1}{k_\mathrm{H}}](images/math/f/3/9/f39b32d825fae72e1bdf768f57e48327.png) where kH=29.76 atm/(mol/L) at 25°C (
where kH=29.76 atm/(mol/L) at 25°C (![\scriptstyle[H^+][OH^-]=10^{-14}](images/math/8/2/0/820d8be050a0a727f6597d89d086a9e9.png) relation and the neutrality condition
relation and the neutrality condition ![\scriptstyle[H^+]=[OH^-]+[HCO_3^-]+2[CO_3^{2-}]](images/math/8/7/9/8799cc0efd127b08f6494b6015e9bc97.png) result in six equations for the six unknowns [CO2], [H2CO3], [H+], [OH−], [HCO3−] and [CO32−], showing that the composition of the solution is fully determined by
result in six equations for the six unknowns [CO2], [H2CO3], [H+], [OH−], [HCO3−] and [CO32−], showing that the composition of the solution is fully determined by 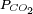 (atm)
(atm)
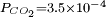 atm), we get a slightly acid solution (pH = 5.7) and the dissolved carbon is now essentially in the CO2 form. From this pressure on, [OH−] becomes also negligible so that the ionized part of the solution is now an equimolar mixture of H+ and HCO3−.
atm), we get a slightly acid solution (pH = 5.7) and the dissolved carbon is now essentially in the CO2 form. From this pressure on, [OH−] becomes also negligible so that the ionized part of the solution is now an equimolar mixture of H+ and HCO3−.
![\scriptstyle[H^+] \simeq \left( 10^{-14}+\frac {K_hK_{a1}}{k_\mathrm{H}} p_{CO_2}\right)^{1/2}](images/math/8/3/1/831ffd4f77d667186b1d24cf7131235e.png)


