To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Diamagnetism
Additional recommended knowledgeDiamagnetism is a weak repulsion from a magnetic field. It is a form of magnetism that is only exhibited by a substance in the presence of an externally applied magnetic field. It results from changes in the orbital motion of electrons. Applying a magnetic field creates a magnetic force on a moving electron in the form of F = Qv × B. This force changes the centripetal force on the electron, causing it to either speed up or slow down in its orbital motion. This changed electron speed modifies the magnetic moment of the orbital in a direction opposing the external field. Consider two electron orbitals; one rotating clockwise and the other counterclockwise. An external magnetic field into the page will make the centripetal force on an electron rotating clockwise increase, causing it to speed up. That same field would make the centripetal force on an electron rotating counterclockwise decrease, causing it to slow down. The orbiting electrons create magnetic fields themselves, and in both cases, the change in B due to the electron's change in velocity is in the opposite direction to the external B field. Since the material originally had no net magnetic field from its orbiting electrons (because their orbits were aligned in random directions), the result is that the induced B field opposes the applied B field, and these repel each other. All materials show a diamagnetic response in an applied magnetic field. In fact, diamagnetism is a very general phenomenon, because all paired electrons, including the core electrons of an atom will always make a weak diamagnetic contribution to the material's response. However, for materials which show some other form of magnetism (such as ferromagnetism or paramagnetism), the diamagnetism is completely overpowered. Substances which only, or mostly, display diamagnetic behaviour are termed diamagnetic materials, or diamagnets. Materials that are said to be diamagnetic are those which are usually considered by non-physicists as "non magnetic", and include water, DNA, most organic compounds such as petroleum and some plastics, and many metals, particularly the heavy ones with many core electrons, such as mercury, gold and bismuth. Diamagnetic materials have a relative magnetic permeability that is less than 1, thus a magnetic susceptibility which is less than 0, and are therefore repelled by magnetic fields. However, since diamagnetism is such a weak property its effects are not observable in every-day life. For example, the magnetic susceptibility of diamagnets such as water is Superconductors may be considered to be perfect diamagnets ( Additionally, all conductors exhibit an effective diamagnetism when they move through a magnetic field. The Lorenz force on electrons causes them to circulate around forming eddy currents. The eddy currents then produce an induced magnetic field which opposes the applied field, resisting the conductors motion. HistoryIn 1778 S. J. Brugmans was the first person to observe that bismuth and antimony were repelled by magnetic fields. However, the term "diamagnetism" was coined by Michael Faraday in September 1845, when he realized that all materials in nature possessed some form of diamagnetic response to an applied magnetic field. Diamagnetic levitation
A particularly fascinating phenomenon involving diamagnets is that they may be levitated in stable equilibrium in a magnetic field, with no power consumption. Earnshaw's theorem seems to preclude the possibility of static magnetic levitation. However, Earnshaw's theory only applies to objects with permanent moments m, such as ferromagnets, whose magnetic energy is given by m·B. Ferromagnets are attracted to field maxima, which do not exist in free space.
Diamagnetism is an induced form of magnetism, thus the magnetic moment is proportional to the applied field B. This means that the magnetic energy of diamagnets is proportional to B², the intensity of the magnetic field. Diamagnets are also attracted to field minima, and there can be a minimum in B² in free space (in fact A thin slice of pyrolytic graphite, which is an unusually strong diamagnetic material, can be stably floated in a magnetic field, such as that from rare earth permanent magnets. This can be done with all components at room temperature, making a visually effective demonstration of diamagnetism. The Radboud University Nijmegen, the Netherlands, has conducted experiments where water and other substances were successfully levitated. Most spectacularly, a live frog (see figure) was levitated.[1] Recent experiments with studying the growth of protein crystals has led to a technique that utilizes powerful magnets to allow growth in ways that counteract Earth's gravity.[2] A simple homemade device may be constructed out of bismuth plates and a few permanent magnets that will levitate a permanent magnet.[citation needed] References
|
||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Diamagnetism". A list of authors is available in Wikipedia. |





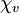 = −9.05×10−6. The most strongly diamagnetic material is
= −9.05×10−6. The most strongly diamagnetic material is 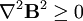 ).
).


