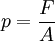To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Diamond anvil cell
A diamond anvil cell (DAC) is a device used to exert extreme pressures on a material consisting of two opposing cone-shaped diamonds squeezed together. The resultant high pressures — in excess of a million atmospheres — are produced when force is applied to small areas of the opposing diamond culets. The device has been used to simulate the extreme pressures existing in the hearts of planets, creating new materials in the process. Notable examples include the non-molecular ice X[1], polymeric nitrogen[2] and MgSiO3 perovskite, thought to be the major component of the Earth's mantle. Additional recommended knowledge
PrincipleThe operation of the diamond anvil cell relies on a simple principle:
where p is the pressure, F the applied force, and A the area. Therefore high pressure can be achieved by applying a moderate force on a sample with a small area, rather than applying a large force on a large area. In order to prevent deformation and even breakage of the anvils that apply the force, they must be made from a very hard and virtually incompressible material, such as diamond. HistoryPercy Williams Bridgman, the great pioneer of high-pressure research during the first half of the 20th century, developed an opposed anvil device with small flat areas that were pressed one against the other with a lever-arm. The anvils were made of a tungsten-carbon alloy (WC). This device could achieve pressure of a few gigapascals, and was used in electrical resistance and compressibility measurements. The revolution in the field of high pressures came with the development of the diamond anvil cell in the late 1950's in the National Bureau of Standards (NBS) by Weir, Lippincott, Van Valkenburg, and Bunting [1]. The principles of the DAC are similar to the Bridgman anvils but in order to achieve the highest possible pressures without breaking the anvils, they were made of the hardest known material: a single crystal diamond. The first prototypes were limited in their pressure range and there was not a reliable way to calibrate the pressure. During the following decades DACs have been successively refined, the most important innovations being the use of gaskets and the ruby pressure calibration. The DAC evolved to be the most powerful lab device for generating static high pressure. The range of static pressure attainable today extends to the estimated pressures at the Earth’s center (~360 GPa). ComponentsThere are many different DAC designs but all have three main components: (1) The force-generating device — relies on the operation of either a lever arm, tightening screws, or gas pressure applied to a membrane. In all cases the force is uniaxial and is applied to the tables (bases) of the two anvils (2) Two opposing diamond anvils — made of high gem quality, flawless diamonds, usually with 16 facets. They typically weigh 1/8 to 1/3 carat (25 to 70 mg). The culet (tip) is ground and polished to a hexadecagonal surface parallel to the table. The culets of the two diamonds face one another, and must be perfectly parallel in order to produce uniform pressure and to prevent dangerous strains. (3) Gasket — a metal foil that separates the two culets. It has an important role: to contain the sample with a hydrostatic fluid in a cavity between the diamonds, and to prevent anvil failure by supporting the diamond tips, thus reducing stresses at the edges of the culet. UsesPrior to the invention of the diamond anvil cell static high-pressure apparatus required large hydraulic presses which weighed several tonnes and required large specialised laboratories. The simplicity and compactness of the DAC mean that it can be accommodated in a wide variety of experiments. Some contemporary DACs can easily fit into a cryostat for low-temperature measurements, and for use with a superconducting electromagnet. In addition to being hard, diamonds have the advantage of being transparent to a wide range of the electromagnetic spectrum from infrared to gamma rays, with the exception of the far ultraviolet and soft X-rays. This makes the DAC a perfect device for spectroscopic experiments and for crystallographic studies using hard X-rays. A variant of the diamond anvil, the hydrothermal diamond anvil cell (HDAC) is used in experimental petrology/geochemistry for the study of aqueous fluids, silicate melts, immiscible liquids, mineral solubility and aqueous fluid speciation at geologic pressures and temperatures. The HDAC is sometimes used to examine aqueous complexes in solution using the synchrotron light source techniques XANES and EXAFS. References
Categories: Materials science | Condensed matter physics | Physical chemistry |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Diamond_anvil_cell". A list of authors is available in Wikipedia. |