To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Dielectric functionThe dielectric function of a material ε(ω) describes its optical properties at all energies Additional recommended knowledgeIn the case of solids, the complex dielectric function is intimately connected to band structure. The primary quantities that characterize the electronic structure of any crystalline material are its critical-point energies. These are the energies, Ei, at which there are discontinuities or infinite first derivatives in the joint density of states or, in the absence of line broadening, in the probability of photon absorption and hence in the imaginary part of the optical dielectric function ε(ω). The optical dielectric function is given by the fundamental expression
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Dielectric_function". A list of authors is available in Wikipedia. |


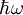 .
.



![\epsilon(\omega)=1+\frac{8\pi^2e^2}{m^2}\sum_{c,v}\int_{}^{} W_{cv}(E) \left[\phi(\hbar\omega-E)-\phi(\hbar\omega+E) \right ] \, dx](images/math/c/d/e/cdec242f9eb40ea4246c8d2421e79247.png) .
In this expression,
.
In this expression, 

