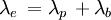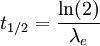To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Effective half-lifeEffective half-life denotes the halving of radioactive material in a living organism by means of radioactive decay and biological excretion. A decay constant is needed to calculate the half-life. It is the sum of the biological and physical decay constants, as in the formula: Additional recommended knowledgeWith the decay constant it is possible to calculate the effective half-life using the formula: The biological decay constant is often approximated as it is more difficult to accurately determine than the physical decay constant.
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Effective_half-life". A list of authors is available in Wikipedia. |