To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electronegativity
The most commonly used method of calculation is that originally proposed by Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale, on a relative scale running from 0.7 to 4.0 (hydrogen = 2.2). When other methods of calculation are used, it is conventional (although not obligatory) to quote the results on a scale that covers the same range of numerical values: this is known as an electronegativity in Pauling units. Electronegativity, as it is usually calculated, is not strictly an atomic property, but rather a property of an atom in a molecule:[3] the equivalent property of a free atom is its electron affinity. It is to be expected that the electronegativity of an element will vary with its chemical environment,[4] but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations. Additional recommended knowledge
Electronegativities of the elements
Methods of calculationPauling electronegativityPauling first proposed[2] the concept of electronegativity in 1932 as an explanation of the fact that the covalent bond between two different atoms (A–B) is stronger than would be expected by taking the average of the strengths of the A–A and B–B bonds. According to valence bond theory, of which Pauling was a notable proponent, this "additional stabilization" of the heteronuclear bond is due to the contribution of ionic canonical forms to the bonding. The difference in electronegativity between atoms A and B is given by: where the dissociation energies, Ed, of the A–B, A–A and B–B bonds are expressed in electronvolts, the factor (eV)−½ being included to ensure a dimensionless result. Hence, the difference in Pauling electronegativity between hydrogen and bromine is 0.73 (dissociation energies: H–Br, 3.79 eV; H–H, 4.52 eV; Br–Br 2.00 eV) As only differences in electronegativity are defined, it is necessary to choose an arbitrary reference point in order to construct a scale. Hydrogen was chosen as the reference, as it forms covalent bonds with a large variety of elements: its electronegativity was fixed first[2] at 2.1, later revised[5] to 2.20. It is also necessary to decide which of the two elements is the more electronegative (equivalent to choosing one of the two possible signs for the square root). This is done by "chemical intuition": in the above example, hydrogen bromide dissolves in water to form H+ and Br− ions, so it may be assumed that bromine is more electronegative than hydrogen. To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element. Allred updated Pauling's original values in 1961 to take account of the greater availability of thermodynamic data,[5] and it is these "revised Pauling" values of the electronegativity which are most usually used. Mulliken electronegativityMulliken proposed that the arithmetic mean of the first ionization energy and the electron affinity should be a measure of the tendency of an atom to attract electrons.[6] As this definition is not dependent on an arbitrary relative scale, it has also been termed absolute electronegativity,[7] with the units of kilojoules per mole or electronvolts. However, it is more usual to make use of a linear transformation to transform these absolute values into values which resemble the more familiar Pauling values. For ionization energies and electron affinities in electronvolts,[8] and for energies in kilojoules per mole,[9] The Mulliken electronegativity can only be calculated for an element for which the electron affinity is known, fifty-seven elements as of 2006. Allred-Rochow electronegativityAllred and Rochow considered[10] that electronegativity should be related to the charge experienced by an electron on the "surface" of an atom: the higher the charge per unit area of atomic surface, the greater the tendency of that atom to attract electrons. The effective nuclear charge, Z* experienced by valence electrons can be estimated using Slater's rules, while the surface area of an atom in a molecule can be taken to be proportional to the square of the covalent radius, rcov. When rcov is expressed in ångströms,
Sanderson electronegativitySanderson has also noted the relationship between electronegativity and atomic size, and has proposed a method of calculation based on the reciprocal of the atomic volume.[11] With a knowledge of bond lengths, Sanderson electronegativities allow the estimation of bond energies in a wide range of compounds.[12] Also Sanderson electronegativities were used for different investigations in organic chemistry.[13][14] Allen electronegativityPerhaps the simplest definition of electronegativity is that of Allen, who has proposed that it is related to the average energy of the valence electrons in a free atom,[15] where εs,p are the one-electron energies of s- and p-electrons in the free atom and ns,p are the number of s- and p-electrons in the valence shell. It is usual to apply a scaling factor, 1.75×10−3 for energies expressed in kilojoules per mole or 0.169 for energies measured in electronvolts, to give values which are numerically similar to Pauling electronegativities. The one-electron energies can be determined directly from spectroscopic data, and so electronegativities calculated by this method are sometimes referred to as spectroscopic electronegativities. The necessary data are available for almost all elements, and this method allows the estimation of electronegativities for elements which cannot be treated by the other methods, e.g. francium, which has an Allen electronegativity of 0.67.[16] However, it is not clear what should be considered to be valence electrons for the d- and f-block elements, which leads to an ambiguity for their electronegativities calculated by the Allen method. Correlation of electronegativity with other propertiesThe wide variety of methods of calculation of electronegativities, which all give results which correlate well with one another, is one indication of the number of chemical properties which might be affected by electronegativity. The most obvious application of electronegativities is in the discussion of bond polarity, for which the concept was introduced by Pauling. In general, the greater the difference in electronegativity between two atoms, the more polar the bond that will be formed between them, with the atom having the higher electronegativity being at the negative end of the dipole. Pauling proposed an equation to relate "ionic character" of a bond to the difference in electronegativity of the two atoms,[3] although this has fallen somewhat into disuse. Several correlations have been shown between infrared stretching frequencies of certain bonds and the electronegativities of the atoms involved:[17] however, this is not surprising as such stretching frequencies depend in part on bond strength, which enters into the calculation of Pauling electronegativities. More convincing are the correlations between electronegativity and chemical shifts in NMR spectroscopy[18] or isomer shifts in Mössbauer spectroscopy[19] (see figure). Both these measurements depend on the s-electron density at the nucleus, and so are a good indication that the different measures of electronegativity really are describing "the ability of an atom in a molecule to attract electrons to itself".[1][3] Trends in electronegativityPeriodic trendsIn general, electronegativity increases on passing from left to right along a period, and decreases on descending a group. Hence, fluorine is undoubtedly the most electronegative of the elements while caesium is the least electronegative, at least of those elements for which substantial data are available.[16] There are some exceptions to this general rule. Gallium and germanium have higher electronegativities than aluminium and silicon respectively because of the d-block contraction. Elements of the fourth period immediately after the first row of the transition metals have unusually small atomic radii because the 3d-electrons are not effective at shielding the increased nuclear charge, and smaller atomic size correlates with higher electronegativity (see Allred-Rochow electronegativity, Sanderson electronegativity above). The anomalously high electronegativity of lead, particularly when compared to thallium and bismuth, appears to be an artifact of data selection (and data availability)—methods of calculation other than the Pauling method show the normal periodic trends for these elements. Variation of electronegativity with oxidation numberIn inorganic chemistry it is common to consider a single value of the electronegativity to be valid for most "normal" situations. While this approach has the advantage of simplicity, it is clear that the electronegativity of an element is not an invariable atomic property and, in particular, increases with the oxidation state of the element. Allred used the Pauling method to calculate separate electronegativities for different oxidation states of the handful of elements (including tin and lead) for which sufficient data was available.[5] However, for most elements, there are not enough different covalent compounds for which bond dissociation energies are known to make this approach feasible. This is particularly true of the transition elements, where quoted electronegativity values are usually, of necessity, averages over several different oxidation states and where trends in electronegativity are harder to see as a result.
The chemical effects of this increase in electronegativity can be seen both in the structures of oxides and halides and in the acidity of oxides and oxoacids. Hence CrO3 and Mn2O7 are acidic oxides with low melting points, while Cr2O3 is amphoteric and Mn2O3 is a completely basic oxide. The effect can also be clearly seen in the dissociation constants of the oxoacids of chlorine. The effect is much larger than could be explained by the negative charge being shared among a larger number of oxygen atoms, which would lead to a difference in pKa of log10(¼) = −0.6 between hypochlorous acid and perchloric acid. As the oxidation state of the central chlorine atom increases, more electron density is drawn from the oxygen atoms onto the chlorine, reducing the partial negative charge on the oxygen atoms and increasing the acidity. Group electronegativityIn organic chemistry, electronegativity is associated more with different functional groups than with individual atoms. The terms group electronegativity and substituent electronegativity are used synonymously. However, it is common to distinguish between the inductive effect and the resonance effect, which might be described as σ- and &pi-electronegativities respectively. There are a number of linear free energy relationships which have been used to quantify these effects, of which the Hammett equation is the best known. Kabachnik parameters are group electronegativities for use in organophosphorus chemistry. See alsoNotes
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electronegativity". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





![\chi_{\rm A} - \chi_{\rm B} = ({\rm eV})^{-1/2} \sqrt{E_{\rm d}({\rm AB}) - [E_{\rm d}({\rm AA}) + E_{\rm d}({\rm BB})]/2}](images/math/d/7/4/d74424c73fed8531b863e9a58ec30f6a.png)
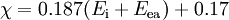
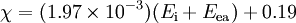
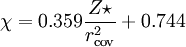 .
.