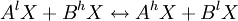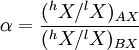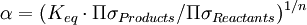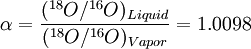To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Equilibrium fractionationEquilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. In general, equilibrium fractionations result from the reduction in vibrational energy (especially zero-point energy) when a more massive isotope is substituted for a less massive one. This leads to higher concentrations of the massive isotopes in substances where the vibrational energy is most sensitive to isotope substitution, i.e., those with the highest bond force constants. Additional recommended knowledgeIn a reaction involving the exchange of two isotopes, lX and hX, of element “X” in molecules AX and BX, each reactant molecule is identical to a product except for the distribution of isotopes (i.e., they are isotopologues). The amount of isotopic fractionation in an exchange reaction can be expressed as a fractionation factor: α = 1 indicates that the isotopes are distributed evenly between AX and BX, with no isotopic fractionation. α > 1 indicates that hX is concentrated in substance AX, and α < 1 indicates hX is concentrated in substance BX. α is closely related to the equilibrium constant (Keq): where ΠσProducts is the product of the rotational symmetry numbers of the products (right side of the exchange reaction), ΠσReactants is the product of the rotational symmetry numbers of the reactants (left side of the exchange reaction), and n is the number of atoms exchanged. An example of equilibrium isotope fractionation is the concentration of heavy isotopes of oxygen in liquid water, relative to water vapor, At 20oC, the equilibrium fractionation factor for this reaction is
See alsoStable isotope ReferencesChacko T., Cole D.R., and Horita J. (2001) Equilibrium oxygen, hydrogen and carbon isotope fractionation factors applicable to geologic systems. Reviews in Mineralogy and Geochemistry, v. 43, p. 1-81. Horita J. and Wesolowski D.J. (1994) Liquid-vapor fractionation of oxygen and hydrogen isotopes of water from the freezing to the critical temperature. Geochimica et Cosmochimica Acta, v. 58, p. 3425-2437. Categories: Isotopes | Geochemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Equilibrium_fractionation". A list of authors is available in Wikipedia. |