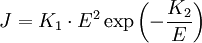To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Fowler-Nordheim equationThe Fowler-Nordheim equation in solid state physics relates current, work and electric field strength to determine field emission. It has two parts: an equation for field emitted current density, and the equation for total current. It is named after Ralph H. Fowler and Lothar W. Nordheim. Additional recommended knowledgeThe current density flowing through a thin oxide layer due to Fowler-Nordheim tunneling is a function of the electric field across the oxide. The electric field is the voltage divided by the distance. This article describes how quickly current increases with voltage.
For the Fowler-Nordheim tunneling current density :
The point is that the current increases with the voltage squared multiplied by an exponential increase with inverse voltage. While the second factor, E², obviously increases rapidly with voltage, the third factor, the exponential, deserves another sentence. For people who are not familiar with exponentials of negative inverses, the following sentences are helpful. Assume, temporarily, that K2 is normalized to be 1. The factor e-1/E increases with E. If E is near zero, the exponent is large, and exp(-large) is near zero. If E is large, 1/E is small, and almost zero: exp(0) = 1 Therefore, exp(-1/E) gets larger as E gets larger, since one is greater than zero. Ultimately, this third factor will maintain a value between zero and one. ConclusionJ increases by two voltage-related factors that are multiplied: (exponentially with voltage) x (squared with voltage)
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Fowler-Nordheim_equation". A list of authors is available in Wikipedia. |