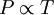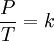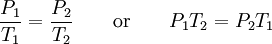To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Gay-Lussac's lawGay-Lussac's law is one of two laws named after the French chemist Joseph Louis Gay-Lussac, which relate to the properties of gases and are known by the same name. Additional recommended knowledgeLaw of combining volumesGay-Lussac's law, known as the law of combining volumes, states that:
Gay-Lussac discovered this law in 1809. This played a major role in the development of modern gas stoichiometry because in 1811, Avogadro used Gay-Lussac’s Law to form Avogadro's hypothesis. Other lawThe other law, discovered in 1802, states that:
It is expressed mathematically as: or where:
This law holds true because temperature is a measure of the average kinetic energy of a substance; as the kinetic energy of a gas increases, its particles collide with the container walls more rapidly, thereby exerting increased pressure. Simply put, if you increase the temperature you increase the pressure. For comparing the same substance under two different sets of conditions, the law can be written as: Charles's Law was also known as the Law of Charles and Gay-Lussac, because Gay-Lussac published the law in 1802 using much of Charles' unpublished data from 1787. However, in recent years the term has fallen out of favor since Gay-Lussac has the second but related law presented here attributed to him. This related form of Gay-Lussac's Law, Charles's Law, and Boyle's law form the combined gas law. The three gas laws in combination with Avogadro's Law can be generalized by the ideal gas law. References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Gay-Lussac's_law". A list of authors is available in Wikipedia. |