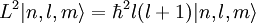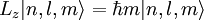To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. It is composed of a single negatively-charged electron circling a single positively-charged proton. The nucleus of hydrogen consists of only a single proton (in the case of hydrogen-1 or protium; see box at right), or it may also include one or more neutrons (giving deuterium, tritium, and other isotopes). The electron is bound to the nucleus by the Coulomb force. This article is primarily about Hydrogen-1, also known as protium or "light hydrogen", which is the primary component of natural hydrogen. The hydrogen atom has special significance in quantum mechanics and quantum field theory as a simple two-body problem physical system which has yielded many simple analytical solutions in closed-form. In 1913, Niels Bohr obtained the spectral frequencies of the hydrogen atom after making a number of simplifying assumptions. These assumptions were not fully correct, but did yield the correct energy answers (see The Bohr Model). Bohr's results for the frequencies and underlying energy values were confirmed by the full quantum-mechanical analysis which uses the Schrödinger equation, as was shown in 1925/26. The solution to the Schrödinger equation for hydrogen is analytical. From this solution, the hydrogen energy levels and thus the frequencies of the hydrogen spectral lines can be calculated. The solution of the Schrödinger equation goes much further than the Bohr model however, because it also yields the shape of the electron's wave function ("orbital") for the various possible quantum-mechanical states-- thus explaining the anisotropic character of atomic bonds. The Schrödinger equation also applies to more complicated atoms and molecules, however, in most such cases the solution is not analytical and either computer calculations are necessary, or else simplifying assumptions must be made. Additional recommended knowledge
Solution of Schrödinger equation: Overview of resultsThe solution of the Schrödinger equation (wave equations) for the hydrogen atom uses the fact that the Coulomb potential produced by the nucleus is isotropic (it is radially symmetric in space and only depends on the distance to the nucleus). Although the resulting energy eigenfunctions (the "orbitals") are not necessarily isotropic themselves, their dependence on the angular coordinates follows completely generally from this isotropy of the underlying potential: The eigenstates of the Hamiltonian (= energy eigenstates) can be chosen as simultaneous eigenstates of the angular momentum operator. This corresponds to the fact that angular momentum is conserved in the orbital motion of the electron around the nucleus. Therefore, the energy eigenstates may be classified by two angular momentum quantum numbers, l and m (integer numbers). The "angular momentum" quantum number l = 0, 1, 2, ... determines the magnitude of the angular momentum. The "magnetic" quantum number m = −l, .., +l determines the projection of the angular momentum on the (arbitrarily chosen) z-axis. In addition to mathematical expressions for total angular momentum and angular momentum projection of wavefunctions, an expression for the radial dependence of the wave functions must be found. It is only here that the details of the 1/r Coulomb potential enter (leading to Laguerre polynomials in r). This leads to a third quantum number, the principal quantum number n = 1, 2, 3, ... The principal quantum number in hydrogen is related to atom's total energy. Note that the maximum value of the angular momentum quantum number is limited by the principal quantum number: it can run only up to n − 1, i.e. l = 0, 1, ..., n − 1. Due to angular momentum conservation, states of the same l but different m have the same energy (this holds for all problems with rotational symmetry). In addition, for the hydrogen atom, states of the same n but different l are also degenerate (i.e. they have the same energy). However, this is a specific property of hydrogen and is no longer true for more complicated atoms which have a (effective) potential differing from the form 1/r (due to the presence of the inner electrons shielding the nucleus potential). Taking into account the spin of the electron adds a last quantum number, the projection of the electron's spin angular momentum along the z axis, which can take on two values. Therefore, any eigenstate of the electron in the hydrogen atom is described fully by four quantum numbers. According to the usual rules of quantum mechanics, the actual state of the electron may be any superposition of these states. This explains also why the choice of z-axis for the directional quantization of the angular momentum vector is immaterial: An orbital of given l and m' obtained for another preferred axis z' can always be represented as a suitable superposition of the various states of different m (but same l) that have been obtained for z. Mathematical summary of eigenstates of hydrogen atomEnergy levelsThe energy levels of hydrogen, including fine structure are given by
The value of -13.6 eV can be found from the simple Bohr model, and is related to the mass, m, and charge of the electron, q: It is even more elegantly connected to fine-structure constant: WavefunctionThe normalized position wavefunctions, given in spherical coordinates are: where:
Angular momentumThe eigenvalues for Angular momentum operator: Visualizing the hydrogen electron orbitalsThe image to the right shows the first few hydrogen atom orbitals (energy eigenfunctions). These are cross-sections of the probability density that are color-coded (black=zero density, white=highest density). The angular momentum quantum number l is denoted in each column, using the usual spectroscopic letter code ("s" means l = 0; "p": l = 1; "d": l = 2). The main quantum number n (= 1, 2, 3, ...) is marked to the right of each row. For all pictures the magnetic quantum number m has been set to 0, and the cross-sectional plane is the xz-plane (z is the vertical axis). The probability density in three-dimensional space is obtained by rotating the one shown here around the z-axis. The "ground state", i.e. the state of lowest energy, in which the electron is usually found, is the first one, the "1s" state (principal quantum leveln = 1, l = 0). An image with more orbitals is also available (up to higher numbers n and l). Note the number of black lines that occur in each but the first orbital. These are "nodal lines" (which are actually nodal surfaces in three dimensions). Their total number is always equal to n − 1, which is the sum of the number of radial nodes (equal to n - l - 1) and the number of angular nodes (equal to l). Features going beyond the Schrödinger solutionThere are several important effects that are neglected by the Schrödinger equation and which are responsible for certain small but measurable deviations of the real spectral lines from the predicted ones:
Both of these features (and more) are incorporated in the relativistic Dirac equation, with predictions that come still closer to experiment. Again the Dirac equation may be solved analytically in the special case of a two-body system, such as the hydrogen atom. The resulting solution quantum states now must be classified by the total angular momentum number j (arising through the coupling between electron spin and orbital angular momentum). States of the same j and the same n are still degenerate.
For these developments, it was essential that the solution of the Dirac equation for the hydrogen atom could be worked out exactly, such that any experimentally observed deviation had to be taken seriously as a signal of failure of the theory. Due to the high precision of the theory also very high precision for the experiments is needed, which utilize a frequency comb. See also
References
Section 4.2 deals with the hydrogen atom specifically, but all of Chapter 4 is relevant.
Categories: Atoms | Hydrogen physics |
|||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Hydrogen_atom". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||





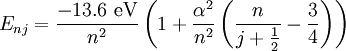
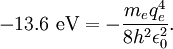
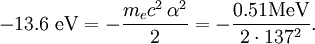
![\psi_{nlm}(r,\vartheta,\varphi) = \sqrt {{\left ( \frac{2}{n a_0} \right )}^3\frac{(n-l-1)!}{2n[(n+l)!]} } e^{- \rho / 2} \rho^{l} L_{n-l-1}^{2l+1}(\rho) \cdot Y_{lm}(\vartheta, \varphi )](images/math/3/5/c/35c9ab655a29fb503262b8daec9585b2.png)
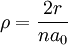
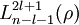 are the generalized Laguerre polynomials of degree n-l-1.
are the generalized Laguerre polynomials of degree n-l-1.
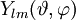 is a
is a