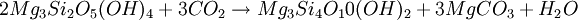To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Magnesite
Magnesite is magnesium carbonate, MgCO3. Iron (as Fe2+) substitutes for magnesium (Mg) with a complete solution series with siderite, FeCO3. Calcium, manganese, cobalt, and nickel may also occur in small amounts. Dolomite, (Mg,Ca)CO3, is almost indistinguishable from magnesite. Additional recommended knowledge
OccurrenceMagnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terranes. These magnesites often are cryptocrystalline and contain silica as opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide within groundwaters. FormationMagnesite can be formed via talc carbonate metasomatism of peridotite and other ultrabasic rocks. Magnesite is formed via carbonation of olivine in the presence of water and carbon dioxide, and is favored at moderate temperatures and pressures typical of greenschist facies; Magnesite can also be formed via the carbonation of magnesian serpentine (lizardite) via the following reaction: Forsterite magnesia-rich olivine compositions favor production of magnesite from peridotite. Fayalitic (iron-rich) olivine favors production of magnetite-magnesite-silica compositions. Magnesite can also be formed from metasomatism in skarn deposits, in dolomitic limestones, associated with wollastonite, periclase, and talc. UsesMagnesite can be used as a slag former in steelmaking furnaces, in conjunction with lime, in order to protect the magnesium oxide lining. It can also be used as a catalyst and filler in the production of synthetic rubber and in the preparation of magnesium chemicals and fertilizers. Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, otherwise known as periclase. Such periclase is an important product in refractory materials. References and external links
Categories: Magnesium minerals | Carbonate minerals |
|||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Magnesite". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||