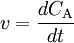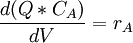To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Mass balanceA mass balance (also called a material balance) is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique. The exact conservation law used in the analysis of the system depends on the context of the problem but all revolve around mass conservation, i.e. that matter cannot disappear or be created spontaneously. Mass balances are used widely in engineering and environmental analyses. For example mass balance theory is used to design chemical reactors, analyse alternative processes to produce chemicals as well as in pollution dispersion models and other models of physical systems. Closely related and complementary analysis techniques include the population balance, energy balance and the somewhat more complex entropy balance. These techniques are required for thorough design and analysis of systems such as the refrigeration cycle. In environmental monitoring the term budget calculations is used to describe mass balance equations where they are used to evaluate the monitoring data (comparing input and output, etc.) In biology the dynamic energy budget theory for metabolic organisation makes explicit use of time, mass and energy balances. Additional recommended knowledge
IntroductionThe general form quoted for a mass balance is The mass that enters a system must, by conservation of mass, either leave the system or accumulate within the system . Mathematically the mass balance for a system without a chemical reaction is as follows: Input = Output + Accumulation In the absence of a chemical reaction the amount of any chemical species flowing in and out will be the same; This gives rise to an equation for each species in the system. However if this is not the case then the mass balance equation must be amended to allow for the generation or depletion of each chemical species. Note that the one term (depletion or generation) is used in the equation, which will be negative for depletion and positive for generation. This modified equation can be used not only for reactive systems, but for population balances such as occur in particle mechanics problems. The amended equation is given below; Note that it simplifies to the earlier equation in the case that the generation term is zero. Input + Generation = Output + Accumulation
Illustrative exampleAt this point a simple example shall be given for illustrative purposes. Consider the situation whereby a slurry is flowing into a settling tank to remove the solids in the tank, solids are collected at the bottom by means of a conveyor belt partially submerged in the tank, water exits via an overflow outlet. In this example we shall consider there to be two species, solids and water. The species are concentrated in each of the output streams, that is to say that the water to solid ratio at the water overflow outlet is higher than at the slurry inlet and the solids concentration at the exit of the conveyor belt is higher than that at the slurry inlet. Assumptions
Analysis The slurry inlet composition has been measured by sampling the inlet and has a composition (by mass) of 50% solid and 50% water, with a mass flow of 100 Kg per minute, the tank is assumed to be operating at steady state, and as such accumulation is zero, so input and output must be equal for both the solids and water. If we know that the removal efficiency for the slurry tank is 60%, then the water outlet will contain 20Kg/min of solids (40% times 100Kg/min times 50% solids). If we measure the flow rate of combined solids and water the water outlet to be 60Kg per minute then the amount of water exiting via the conveyor belt is 10Kg/min. This allows us to completely determine how the mass has been distributed in the system with only limited information and using the mass balance relations across the system boundaries Mass Feedback (Recycle)Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit for often for further reprocessing. Such systems are common in grinding circuits, where materials are crushed then sieved to only allow a particular size of particle out of the circuit and the larger particles are returned to the grinder. However recycle flows are by no means restricted to solid mechanics operations, they are used in liquid and gas flows as well. One such example is in cooling towers, where water is pumped through the cooling tower many times, with only a small quantity of water drawn off at each pass (to prevent solids build up) until it has either evaporated or exited with the drawn off water. The use of the recycle aids in increasing overall conversion of input products, which is useful for low per-pass conversion processes, for example the Haber process. Differential Mass balancesA mass balance can also be taken differentially. The concept is the same as for a large mass balance, however it is performed in the context of a limiting system (for example, one can consider the limiting case in time or, more commonly, volume). The use of a differential mass balance is to generate differential equations that can be used to provide an understanding and effective modelling tool for the target system. The differential mass balance is usually solved in two steps, firstly a set of governing differential equations must be obtained, and then these equations must be solved, either analytically or, for less tractable problems, numerically. A good example of the applications of differential mass balance are shown in the following systems:
Ideal Batch reactorA closed system. Many chemistry textbooks implicitly assumes that the studied system can be described as a batch reactor when they write about reaction kinetics and chemical equilibrium The mass balance for a substance A becomes IN + PROD = OUT + ACC
where r_A denotes the rate at which substance A is produced, V is the volume (which may be constant or not), n_A the number of moles (n) of substance A. In a fed-batch reactor some reactants/ingredients are added continuously or in pulses (compare making porridge by either first blending all ingredients and the let it boil, which can be described as a batch reactor, or by first mixing only water and salt and making that boil before the other ingredients are added, which can be described as a fed-batch reactor). Mass balances for fed-batch reactors become a bit more complicated. Reactive ExampleIn this example we will use the law of mass action to derive the expression for a chemical equilibrium constant. Assume we have a closed reactor in which the following liquid phase reversible reaction occurs:
The mass balance for substance A becomes IN + PROD = OUT + ACC
As we have a liquid phase reaction we can (usually) assume a constant volume and since nA = V * CA we get
or
In many text books this is given as the definition of reaction rate without specifying the implicit assumption that we are talking about reaction rate in a closed system with only one reaction. This is an unfortunate mistake that has confused many students over the years. According to the law of mass action the forward reaction rate can be written as r1 = k1[A]a[B]b and the backward reaction rate as r − 1 = k − 11[C]c[D]d The rate at which substance A is produced is thus rA = r − 1 − r1 and since, at equilibrium, the concentration of A is constant we get
or, rearranged
Ideal tank reactor/Continuously stirred tank reactorAn open system. A lake can be regarded as a tank reactor and lakes with long turnover times (e.g. with a low flux to volume ratio) can for many purposes be regarded as continuously stirred (e.g. homogeneous in all respects). The mass balance becomes IN + PROD = OUT + ACC
where Q_0 and Q denote the volumetric flow in and out of the system respectively and C_A_0 and C_A the concentration of A in the inflow and outflow respective. In an open system we can never reach a chemical equilibrium. We can, however, reach a steady state where all state variables (temperature, concentrations etc.) remain constant (ACC = 0) ExampleConsider a bathtub in which we have some bathing salt dissolved. We now fill in more water, keeping the bottom plug in. What happens? Since there is no reaction, PROD = 0 and since there is no outflow Q = 0. The mass balance becomes IN + PROD = OUT + ACC
or
Using a mass balance for total volume, however, it is evident that
Note that there is no reaction and hence no reaction rate or rate law involved, and yet
without mentioning that this definition implicitly assumes that the system is closed, has a constant volume and that there is only one reaction.written by bobby Ideal Plug Flow Reactor (PFR)An open system with no mixing along the reactor but perfect mixing across the reactor. Often used for systems like rivers and water pipes if the flow is turbulent. When a mass balance is made for a tube, one first considers an infinitesimal part of the tube and make a mass balance over that using the ideal tank reactor model. That mass balance is then integrated over the entire reactor volume to obtain:
In numeric solutions, e.g. when using computers, the ideal tube is often translated to a series of tank reactors, as it can be shown that a PFR is equivalent to an infinite number of stirred tanks in series, but the latter is often easier to analyze, especially at steady state. More complex problemsIn reality, reactors are often non-ideal, in which combinations of the reactor models above are used to describe the system. Not only chemical reaction rates, but also mass transfer rates may be important in the mathematical description of a system, especially in heterogeneous systems. As the chemical reaction rate depends on temperature it is often necessary to make both an energy balance (often a heat balance rather than a full fledged energy balance) as well as mass balances to fully describe the system. A different reactor models might be needed for the energy balance: A system that is closed with respect to mass might be open with respect to energy e.g. since heat may enter the system through conduction. See also
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Mass_balance". A list of authors is available in Wikipedia. |





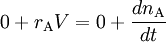
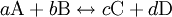
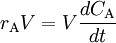
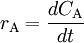
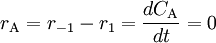
![\frac{k_1}{k_{-1}}=\frac{[\mathrm{C}]^c[\mathrm{D}]^d}{[\mathrm{A}]^a[\mathrm{B}]^b}=K_{eq}](images/math/c/9/0/c90c83612b269d8faab12714258c4cdd.png)
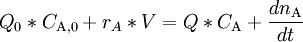
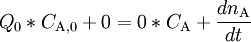
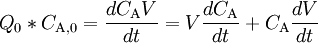
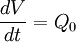 and that
and that 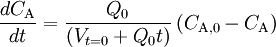
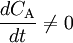 . We can thus draw the conclusion that reaction rate can not be defined in a general manner using
. We can thus draw the conclusion that reaction rate can not be defined in a general manner using 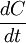 . One must first write down a mass balance before a link between
. One must first write down a mass balance before a link between