To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Mercury coulometerMercury coulometer is based on the electrochemical processes of oxidation/reduction with according of the follow reaction: Additional recommended knowledgeConstructionThis coulometer has different constructions but all of them are based on mass measurements. One of the most significant construction is shown on the picture. It consists of two reservoirs connected by a thin graduated capillary. All system contains solution of the mercury(II)-ions solution. Each of the reservoirs have an electrode immersed into a drop of mercury. Another small drop of mercury is inserted into the capillary. When the current is turned on, it will initiate dissolution of the metallic mercury on the one side of the drop in the capillary and deposition on the other side of the same drop. This drop starts to move. Because of the 100% efficiency of the deposition/dissolution of the mercury under the current influence, mass or volume of this small drop will be a constant and its movement will be lineary correlated with the passed charge. If you change direction of the current, the drop starts move in opposite direction. Sensitivity of this type of coulometers depends on the diameter of the capillary. See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Mercury_coulometer". A list of authors is available in Wikipedia. |


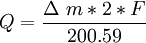 ,
,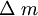 -mass changes (gr); F-Faraday constant (96485);
-mass changes (gr); F-Faraday constant (96485);





