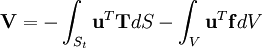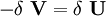To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Minimum total potential energy principleThe principle of minimum total potential energy is a fundamental concept used in physics, chemistry, biology, and engineering. It asserts that a structure or body shall deform or displace to a position that minimizes the total potential energy, with the lost potential energy being dissipated as heat. For example, a marble placed in a bowl will move to the bottom and rest there, and similarly, a tree branch laden with snow will bend to a lower position. The lower position is the position for minimum potential energy: it is the stable configuration for equilibrium. The principle has many applications in structural analysis and solid mechanics. Additional recommended knowledgeThe tendency to minimum total potential energy is due to the second law of thermodynamics, which states that the entropy of a system will maximize at equilibrium. Given two possibilities - a low heat content and a high potential energy, or a high heat content and low potential energy, the latter will be the state with the highest entropy, and will therefore be the state towards which the system moves. The principle of minimum total potential energy should not be confused with the related principle of minimum energy which states that for a system that changes without heat transfer, the total energy will be minimized. Note that in most complex systems there is one global minimum and many local minima (smaller dips) in the potential energy. These are called metastable states. A system may reside in a local minimum for a long time — even an effectively infinite period of time. Some examples
Structural MechanicsThe total potential energy, This energy is at a stationary position when an infinitesimal variation from such position involves no change in energy: The principle of minimum total potential energy may be derived as a special case of the virtual work principle for elastic systems subject to conservative forces. The equality between external and internal virtual work (due to virtual displacements) is: where
In the special case of elastic bodies, the right-hand-side of (3) can be taken to be the change, where the minus sign implies a loss of potential energy as the force is displaced in its direction. With these two subsidiary conditions, (3) becomes: This leads to (2) as desired. The variational form of (2) is often used as the basis for developing the finite element method in structural mechanics. Categories: Thermodynamics | Solid mechanics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Minimum_total_potential_energy_principle". A list of authors is available in Wikipedia. |





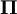 , is the sum of the elastic strain energy, U, stored in the deformed body and the potential energy, V, of the applied forces:
, is the sum of the elastic strain energy, U, stored in the deformed body and the potential energy, V, of the applied forces:
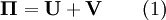
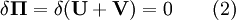
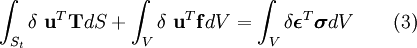
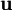 = vector of displacements
= vector of displacements
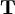 = vector of distributed forces acting on the part
= vector of distributed forces acting on the part 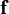 = vector of body forces
= vector of body forces
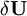 , of elastic strain energy U due to infinitesimal variations of real displacements.
In addition, when the external forces are conservative forces, the left-hand-side of (3) can be seen as the change in the potential energy function V of the forces. The function V is defined as:
, of elastic strain energy U due to infinitesimal variations of real displacements.
In addition, when the external forces are conservative forces, the left-hand-side of (3) can be seen as the change in the potential energy function V of the forces. The function V is defined as: