To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Molar conductivityMolar conductivity is defined as the conductivity of an electrolyte solution divided by the molar concentration of the electrolyte, and so measures the efficiency with which a given electrolyte conducts electricity in solution. Its units are siemens per meter per molarity, or siemens meter-squared per mole. The usual symbol is a capital lambda, Λ, or Λm. Additional recommended knowledgeHistoryFriedrich Kohlrausch established that to a high accuracy in dilute solutions, molar conductivity is composed of individual contributions of ions. This is known as the law of independent migration of ions.[1] DescriptionFrom its definition, the molar conductivity is given by[2]: where
For strong electrolytes, such as salts, strong acids and strong bases, molar conductivity is only weakly dependent on concentration and, to a good approximation, fits to[3] where
In contrast, Kohlrausch showed that the molar conductivity is strongly concentration dependent for weak (incompletely dissociated) electrolytes; the more dilute a solution, the greater its molar conductivity, due to increased ionic dissociation. (This, for example, is the case of proteins in polyacrylamide gel electrophoresis.) The limiting molar conductivity can be decomposed into contributions from the different ions (law of independent migration of ions): where
References
Categories: Electrochemistry | Physical chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Molar_conductivity". A list of authors is available in Wikipedia. |





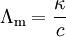
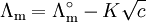
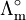 is the molar conductivity at infinite dilution (or limiting molar conductivity)
is the molar conductivity at infinite dilution (or limiting molar conductivity)