To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
NitrosoniumThe nitrosonium ion is NO+, the nitrogen atom is bonded to an oxygen atom with a bond order of 3, the overall diatomic species bearing a positive charge. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosyl sulfuric acid, more descriptively written ONSO2OH), and NOBF4. The ClO4− and BF4− salts are slightly soluble in CH3CN. NOBF4 can be purified by sublimation at 200–250 °C/0.01 mmHg. For NOBF4: Selected data: density = 2.185 g cm–3.3 MW =116.82 NO+ is isoelectronic with CO and N2. It arises via protonation of nitrous acid:
Additional recommended knowledge
Chemical PropertiesHydrolysisNO+ reacts readily with water to form nitrous acid:
For this reason, NOBF4 must be protected from water or even moist air. With base, the reaction generates nitrite:
As a diazotizing agentNO+ reacts with aryl amines, ArNH2, to give diazonium salt, ArN2+. This is useful because N2+ is a more readily leaving group than NH2.
As an oxidizing agentNO+, e.g. as NOBF4, is a strong oxidizing agent:
NOBF4 is a convenient oxidant because the byproduct NO is a gas, which can be swept from the reaction using a stream of N2. Upon contact with air, NO forms NO2, which can cause secondary reactions if it is not removed. NO2 is readily detectable by its characteristic orange color. Nitrosylation of arenesElectron-rich arenes are nitrosylated using NOBF4. The example involves anisole:
Nitrosonium, NO+, is sometimes confused with nitronium, NO2+, the active agent in nitrations. These species are quite different, however. Nitronium is a more potent electrophile than is nitrosonium, as anticipated by the fact that the former is derived from a strong acid (nitric acid) and the latter from a weak acid (nitrous acid). As a source of NO complexesNOBF4 reacts with some metal carbonyl complexes to yield related metal nitrosyl complexes. One must be careful that [NO]+ is transferred vs. electron transfer (see above).
See alsoReferences
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Nitrosonium". A list of authors is available in Wikipedia. |


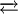 NO+ + H2O
NO+ + H2O





