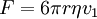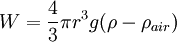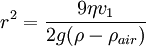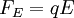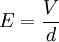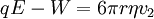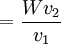To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Oil-drop experiment
Additional recommended knowledge
BackgroundStarting in 1909, while a professor at the University of Chicago, Millikan, with the significant input of Fletcher, worked on the oil-drop experiment (since repeated, with varying degrees of success, by generations of physics students) in which he measured the charge on a single electron. After a publication on his first results [1] in 1910, contradictory observations by Felix Ehrenhaft [2] started a controversy between the two physicists. After improving his setup he published his seminal study in 1913. [3] The so-called elementary charge is one of the fundamental physical constants and accurate knowledge of its value is of great importance. His experiment measured the force on tiny charged droplets of oil suspended against gravity between two metal electrodes. Knowing the electric field, the charge on the droplet could be determined. Repeating the experiment for many droplets, Millikan showed that the results could be explained as integer multiples of a common value (1.592 × 10−19 coulomb), the charge on a single electron. Although at the time of Millikan and Fletcher's oil drop experiments it was becoming clear that there exist such things as subatomic particles, not everyone was convinced. Experimenting with cathode rays in 1897, J.J. Thompson had discovered negatively charged "corpuscles", as he called them, with a mass about 1000 times smaller than that of a hydrogen atom. Similar results had been found by George FitzGerald and Walter Kaufmann. Most of what was then known about electricity and magnetism, however, could be explained on the basis that charge is a continuous variable; in much the same way that many of the properties of light can be explained by treating it as a continuous wave rather than as a stream of photons. The beauty of the oil drop experiment is that as well as allowing quite accurate determination of the fundamental unit of charge Millikan and Fletcher's apparatus also provides a "hands on" demonstration that charge is actually quantized. It demonstrates this simply and elegantly. Thomas Edison, who had previously thought that charge is a continuous variable, became convinced after having a go with Millikan and Fletcher's apparatus. There is some controversy over the use of selectivity in Millikan's results of his second experiment measuring the electron charge raised by the historian Gerald Holton. Holton (1978) pointed out that Millikan disregarded a large set of the oil-drops gained in his experiments without apparent reason. Allan Franklin, a former high energy experimentalist and current philosopher of science at the University of Colorado has tried to rebut this point by Holton[4]. Franklin contends that Millikan's exclusions of data did not affect the final value of e that Millikan obtained but concedes that there was substantial "cosmetic surgery" that Millikan performed which had the effect of reducing the statistical error on e. This enabled Millikan to quote the figure that he had calculated e to better than one half of one percent; in fact, if Millikan had included all of the data he threw out, it would have been to within 2%. While this would still have resulted in Millikan having measured e better than anyone else at the time, the slightly larger uncertainty might have allowed more disagreement with his results within the physics community, which Millikan likely tried to avoid. In 1923, Millikan won the Nobel Prize for physics in part because of this experiment. This experiment has since been repeated by generations of physics students, although it is rather expensive and difficult to do properly. Experimental procedure
The apparatusRobert Millikan’s design is just a uniform electric field, which is a pair of parallel plates that lie horizontal with large potential difference. Then the oil drops are dropped in to the plates and the drops are suspended between the plates. By changing the voltage you can make the oil drops rise and fall. A ring of insulating material is used to hold the plates together. The plates have four holes cut into it and three have a bright light shining through them, and the other has a microscope placed through it. The oil is a type that is usually used in vacuum apparatus. This is because this type of oil has an extremely low vapour pressure. Ordinary oil would evaporate away under the heat of the light source, so the mass of the oil drop would not remain constant over the course of the experiment. Some oil drops will pick up a charge through friction with the nozzle as they are sprayed, but more can be charged by including an ionising radiation source (such as an X-ray tube). MethodInitially the oil drops are allowed to fall between the plates with the electric field turned off. They very quickly reach a terminal velocity because of friction with the air in the chamber. The field is then turned on and, if it is large enough, some of the drops (the charged ones) will start to rise. (This is because the upwards electric force FE is greater for them than the downwards gravitational force W, in the same way bits of paper can be picked up by a charged rubber rod.) A likely looking drop is selected and kept in the middle of the field of view by alternately switching off the voltage until all the other drops have fallen. The experiment is then continued with this one drop. The drop is allowed to fall and its terminal velocity v1 in the absence of an electric field is calculated. The drag force acting on the drop can then be worked out using Stokes' law:
The weight W is the volume V multiplied by the density ρ and the acceleration due to gravity g. However, what is needed is the apparent weight. The apparent weight in air is the true weight minus the upthrust (which equals the weight of air displaced by the oil drop). For a perfectly spherical droplet the apparent weight can be written as: Now at terminal velocity the oil drop is not accelerating. So the total force acting on it must be zero. So the two forces F and W must cancel one another out. Once r is calculated, W can easily be worked out. Now the field is turned back on, and the electric force on the drop is where q is the charge on the oil drop and E is the electric field between the plates. For parallel plates where V is the potential difference and d is the distance between the plates. One conceivable way to work out q would be to adjust V until the oil drop remained steady. Then we could equate FE with W. But in practice this is extremely difficult to do precisely. A more practical approach is to turn V up slightly so that the oil drop rises with a new terminal velocity v2. Then Millikan's experiment and cargo cult scienceRichard Feynman said in a commencement lecture he gave at Caltech in 1974[5] We have learned a lot from experience about how to handle some of the ways we fool ourselves. One example: Millikan measured the charge on an electron by an experiment with falling oil drops, and got an answer which we now know not to be quite right. It's a little bit off because he had the incorrect value for the viscosity of air. It's interesting to look at the history of measurements of the charge of an electron, after Millikan. If you plot them as a function of time, you find that one is a little bit bigger than Millikan's, and the next one's a little bit bigger than that, and the next one's a little bit bigger than that, until finally they settle down to a number which is higher. Why didn't they discover the new number was higher right away? It's a thing that scientists are ashamed of - this history - because it's apparent that people did things like this: When they got a number that was too high above Millikan's, they thought something must be wrong - and they would look for and find a reason why something might be wrong. When they got a number close to Millikan's value they didn't look so hard. And so they eliminated the numbers that were too far off, and did other things like that. We've learned those tricks nowadays, and now we don't have that kind of a disease. As of 2006, the accepted value for the elementary charge is 1.60217653(14) x 10−19 coulombs,[6] where the 14 indicates the uncertainty of the last two decimal places. In his Nobel lecture, Millikan gave his measurement as 4.774(5) x 10−10 statcoulombs,[7] which equals 1.5924(17) x 10−19 coulombs. The difference is less than one percent, but it is more than five times greater than Millikan's standard error, so the disagreement is significant. Notes
Further reading
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Oil-drop_experiment". A list of authors is available in Wikipedia. |