To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Pyramidal alkenePyramidal alkenes are alkenes in which the two carbon atoms making up the double bond are not coplanar with their four substituents . This deformation from a trigonal planar geometry to a tetrahedral molecular geometry is the result of angle strain induced in the molecule due to geometric constraints. Pyramidal alkenes only exist in the laboratory but are of interest because much can be learned from them about the nature of chemical bonding [1]. Additional recommended knowledgeIn cycloheptene (1.1) the cis isomer is an ordinary unstrained molecule but the heptane ring is too small to accommodate a trans configured alkene group resulting in strain and twisting of the double bond. The p-orbital misalignment is minimized by a degree of pyramidalization. In the related anti-bredt molecules it is not pyrimidalization but twisting that dominates. Pyramidalized cage alkenes also exist where symmetrical bending of the substituents predominates without p-orbital misalignment. The pyramidalization angle the butterfly bending angle or folding angle In alkenes 1.2 and 1.3 these angles are determined with x-ray crystallography as respectively 32.4°/22.7° and 27.3°/35.6°. Although stable, these alkenes are very reactive compared to ordinary alkenes. They are liable to dimerization to cyclobutadiene compounds or react with oxygen to epoxides. The compound tetradehydrodianthracene also with a 35° pyramidalization angle is synthesized in a photochemical cycloaddition of bromoanthracene followed by elimination of hydrogen bromide This compound is very reactive in Diels-Alder reactions due to through-space interactions between the two alken groups. This enhanced reactivity enabled in turn the synthesis of the first-ever Möbius aromat. In one study [2] the strained alkene 3.4 was synthesized with the highest pyramidalizion angles yet, 33.5° and 34.3°. This compound is the double Diels-Alder adduct of the diiodide-cyclophane 3.1 and anthracene 3.3 by reaction in presence of potassium tert-butoxide in refluxing dibutyl ether through a di-aryne intermediate 3.2. This is a stable compound but will slowly react with oxygen to an epoxide when left standing as a chloroform solution. In one study [3] , isolation of a pyramidal alkene is not even possible in by matrix isolation at extremely low temperatures unless stabilized by metal coordination: A reaction of the di-iodide 4.1 in fig. 4 with sodium amalgam in the presence of ethylenebis(triphenylphosphine)platinum(0) does not give the intermediate alkene 4.2 but the platinum stabilized 4.3. The sigma bond in this compound is destroyed in reaction with ethanol. References
Categories: Alkenes | Chemical bonding |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Pyramidal_alkene". A list of authors is available in Wikipedia. |





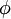 (b) is defined as the angle between the plane defined by one of the doubly bonded carbons and its two substituents and the extension of the double bond and is calculated as:
(b) is defined as the angle between the plane defined by one of the doubly bonded carbons and its two substituents and the extension of the double bond and is calculated as:
![\ cos\phi\ = -cos(R-C-C)/[cos(1/2(R-C-R))]](images/math/6/1/3/6132e826d2d30764a80680956a59e2a8.png)
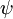 (c) is defined as the angle between two planes and can be obtained by averaging the two
(c) is defined as the angle between two planes and can be obtained by averaging the two 

