To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Quantum concentrationThe quantum concentration nQ is the particle concentration (i.e. the number of particles per unit volume) of a system where the interparticle distance is equal to the thermal de Broglie wavelength or equivalently when the wavefunctions of the particles are touching but not overlapping. Additional recommended knowledgeQuantum effects become appreciable when the particle concentration is greater than or equal to the quantum concentration, which is defined as:
As the quantum concentration depends on temperature; high temperatures will put most systems in the classical limit unless they have a very high density e.g. a White dwarf. |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Quantum_concentration". A list of authors is available in Wikipedia. |





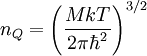
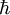 is the reduced Planck constant
is the reduced Planck constant


