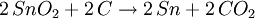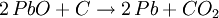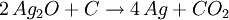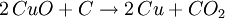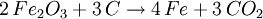To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Smelting
Chemical reduction, or smelting, is a form of extractive metallurgy. The main use of smelting is to produce a metal from its ore. This includes iron extraction (for the production of steel) from iron ore, and copper extraction and other base metals from their ores. It makes use of a chemical reducing agent, commonly a fuel that is a source of carbon such as coke, or in earlier times charcoal, to change the oxidation state of the metal ore; however, plants for the electrolytic reduction of aluminum are also generally referred to as smelters. The carbon or carbon monoxide derived from it removes oxygen from the ore to leave the metal. The carbon is oxidised, producing carbon dioxide and carbon monoxide. As most ores are impure, it is often necessary to use flux, such as limestone to remove the accompanying rock gangue as slag (also called scoria or cinder). Additional recommended knowledge
Smelting basicsThe 7 metals that were known in ancient times (mercury, tin, lead, copper, silver, gold, and iron) can in principle be smelted through similar chemical reactions from their ores:
Different ores require different reactions at different temperatures, but almost always the reducing agent is carbon. The list above is sorted in increasing temperature order, so iron is the most difficult metal to smelt from the ones in the list (that's why historically iron smelting was the last to be discovered). A common mistake is to think that the metal is obtained from the ore because at high temperature the metal just melts out of the ore. That is incorrect: if a blacksmith just heats up the ore without the proper reducing agent (carbon), he will just obtain molten ore. Also, one can smelt some ores at a temperature lower than the temperature required to melt the metal. Usually, though, these reactions happen at temperatures high enough to melt the resulting metal, so the metal can just be cast directly out of the furnace. The exception to the previous paragraph is that some metal oxides just decompose at relatively low temperatures, so instead of trying to smelt mercury out of mercury oxide, one can just heat up mercury oxide to about 500°C, and the oxide will decompose into mercury and oxygen; as mercury boils at 357C, this will cause the oxide to decompose and boil out, producing the highly toxic gaseous mercury. This is possible only for mercury and a handful of other metal oxides; most metal oxides must be smelt with carbon as the reducing agent. First smelting: campfiresSmelting is a chemical reaction that requires a particular ore (that sometimes look like any other common sedimentary rock), a particular content of carbon and a particular temperature in order to produce the metal. Without knowledge of chemistry, it is impossible to predict if a given rock can be smelted or not, and what it will produce. Therefore, there is continuous debate to understand how the ancient people learned how to smelt. Probably the first smelting was done by accident by making a campfire on top of tin or lead ores. That may accidentally produce metallic tin and lead at the bottom of the campfire, as the temperatures to smelt tin and lead are easily obtained in a campfire. These metals can then be molten and cast in a campfire. The earliest cast lead beads known today were found in the Çatal Höyük site in Anatolia (Turkey), and were dated of 6500BC. It is unclear when the earliest cast tin artifacts were made, given that tin is much more uncommon than lead, and earlier tin artifacts may have been reused to make bronze. Although lead is a relatively common metal, it is too soft to be of much utility, so the first smelting of lead didn't have significant impact in the ancient world. Copper smelting: kilnsThere were in the past some arguments that copper was first smelt by accident also in campfires, but that seems improbable as campfires are about 200°C short of the temperature needed to smelt copper. A more probable path may have been through pottery kilns, invented in Iran by 6000BC. Pottery kilns produce ceramics that can be glazed with colorful earths (mostly metallic oxides) to produce colorful vases; it happens that malachite (copper oxide) is a colorful green stone, so a potter that encrusts malachite in a ceramic vase in a coal-fired kiln will produce a few droplets of metallic copper (ruining his vase). That may have set the way to smelt copper. The first known cast copper artifact is a mace head found in Can Hasan from 5000BC. Copper created some impact on the ancient world, as it produces good blunt weapons and reasonable armor, but it is still too soft to produce useful blade weapons. Therefore, the smelting of copper did not replace the manufacture of stone weapons, which still produced superior blades. Bronze smeltingBronze is a copper/arsenic or copper/tin alloy. The presence of arsenic and tin dramatically increased the hardness of copper and produced war-winning weapons, as a bronze mace or hammer seemed indestructible by then, as compared to stone maces and hammers that frequently shattered and flaked on impact. When smiths learned to make bronze daggers and swords they found that they kept their edge much longer compared to the existing stone and volcanic glass daggers. Moreover, while one cannot make stone armor (and therefore warriors had to rely on leather armor), bronze can be readily made into a body armor which is impervious to all weapons of the period. Therefore, knowledge of the smelting of bronze allowed kings to overcome their enemies, and caused such a revolution that it marked the end of the Stone Age and the beginning of the Bronze Age. It would be millennia, though, until bronze could be used by common soldiers and townsfolk, and for a long time they were luxury items used by nobility. The first copper/arsenic bronzes date of 4200BC from Asia Minor, and were used for a long time until replaced by the modern copper/tin bronzes by 1500BC. It is unclear that if at some point in time the smiths that produced copper/arsenic bronze added arsenic oxides on purpose, or if they explored some copper lodes that happened to have arsenic as a lucky contamination. The first copper/tin bronzes date of 3200BC, again from Asia Minor. Copper/tin bronzes are harder and more durable than copper/arsenic ones, and made these obsolete. The process through which the smiths learned to produce copper/tin bronzes is once again a mystery. The first such bronzes were probably a lucky accident from tin contamination of copper ores, but by 2000BC we know that tin was being mined on purpose for the production of bronze. This is amazing, given that tin is a semi-rare metal, and even a rich cassiterite ore only has 5% tin. Also, cassiterite looks like any common rock, and it takes special skills (or special instruments) to find it and locate the richer lodes. But, whatever steps were taken to learn about tin, these were fully understood by 2000BC. Early iron smeltingThe earliest evidence to date for the bloomery smelting of iron is found at Tell Hammeh, Jordan (see also external link), and dates to 930 CalBC (C14 dating). However, based on the archaeological record of iron artifacts, it is clear that intentional reduction of iron metal from terrestrial ores (in the case of Hammeh a Haematite ore), must have started near the end of the Late Bronze Age (ca. 1600–1150 BC). Where and how iron smelting was discovered is widely debated, and remains uncertain due to the significant lack of production finds. Nevertheless, there is some consensus that iron technology originated in the Near East, perhaps in Eastern Anatolia. In Ancient Egypt somewhere between the Third Intermediate Period and 23rd Dynasty (ca. 1100–750 BC) there are indications of iron working. Significantly though, no evidence for the smelting of iron from ore has been attested to in Egypt in any period. There are further indications of iron smelting and working in West Africa by 1200 BC[1]. In addition, very early instances of carbon steel was found to be in production around 2000 YBP in northwest Tanzania, based on complex preheating principles. These discoveries are significant for the history of metallurgy.[2] Most early processes in Europe and Africa involved smelting iron ore in a bloomery, where the temperature is kept low enough so that the iron does not melt. This produces a spongy mass of iron called a bloom, which then has to be consolidated with a hammer. Later iron smeltingFrom the medieval period, the process of direct reduction in bloomeries began to be replaced by an indirect process. In this a blast furnace was used to make pig iron, which then had to undergo a further process to make forgeable bar iron. Further details of this will be found in the article on the blast furnace. Processes for the second stage include fining in a finery forge and from the Industrial Revolution puddling. However both processes are now obsolete, and wrought iron is now hardly made. Instead, mild steel is produced from a bessemer converter or by other means. Base metalsThe ores of base metals are often sulphides. In recent centuries, reverberatory smelters (sometimes called cupolas) have been used. These keep the fuel and the charge being smelted separate. Traditionally these were used for carrying out the first step: formation of two liquids, one an oxide slag containing most of the impurity elements, and the other a sulfide matte containing the valuable metal sulfide and some impurities. Such "reverb" furnaces are today about 40 m long, 3 m high and 10 m wide. Fuel is burned at one end and the heat melts the dry sulfide concentrates (usually after partial roasting), which is fed through the openings in the roof of the furnace. The slag floats on top of the heavier matte, and is removed and discarded or recycled. The sulfide matte is then sent to the converter. However the precise details of the process will vary for one metal to another. References
BibliographyPleiner, R. (2000) Iron in Archaeology. The European Bloomery Smelters, Praha, Archeologický Ústav Av Cr. See also
Categories: Metallurgy | Metals processes | Steelmaking |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Smelting". A list of authors is available in Wikipedia. |