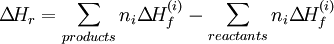To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Standard enthalpy change of reactionThe standard enthalpy change of reaction (denoted ΔH° or ΔH Additional recommended knowledgeFor a generic chemical reaction
the standard enthalpy change of reaction ΔHr is related with the standard enthalpy change of formation ΔHf of the reactants and products by the following equation:
In this equation, the ni corresponds to the stoichiometric coefficients. Reactions with standard valuesA common standard enthalpy change is the standard enthalpy change of formation, which has been determined for a vast number of substances. The enthalpy change of any reaction under any conditions can be computed, given the standard enthalpy change of formation of all of the reactants and products. Other reactions with standard enthalpy change values include combustion (standard enthalpy change of combustion), neutralisation (standard enthalpy change of neutralisation), and solution (standard enthalpy change of solution). Categories: Enthalpy | Chemical reactions | Chemical engineering |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Standard_enthalpy_change_of_reaction". A list of authors is available in Wikipedia. |