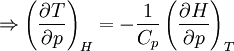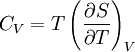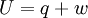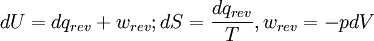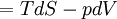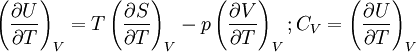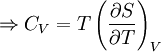To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Table of thermodynamic equations
The following page is a concise list of common thermodynamic equations and quantities: Additional recommended knowledge
List
The following equations are classified by subject. First law of thermodynamicsNote that the symbol δ represents the fact that because q and w are not state functions, δq and δw are inexact differentials. In some fields such as physical chemistry, positive work is conventionally considered work done on the system rather than by the system, and the law is expressed as dU = δq + δw. Entropy
Quantum Properties
Quasi-static and reversible processHeat capacity at constant pressureHeat capacity at constant volumeEnthalpyHelmholtz free energyGibbs free energyMaxwell relations
Incremental processesCompressibility at constant temperatureMore relations
Equation Table for an Ideal Gas (PVm = constant)
Other useful identities
Proof #1An example using the above methods is:
Proof #2Another example:
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Table_of_thermodynamic_equations". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





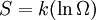
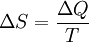 , only for reversible processes
, only for reversible processes
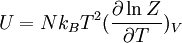
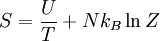 Distinguishable Particles
Distinguishable Particles
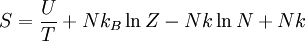 Indistinguishable Particles
Indistinguishable Particles
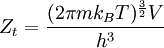
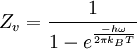
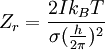
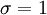 heteronuclear
heteronuclear
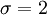 homonuclear
homonuclear
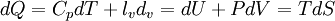
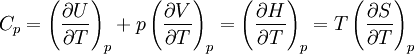
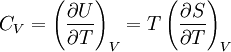
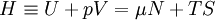
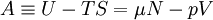
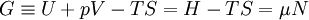
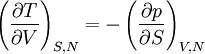
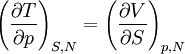
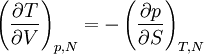
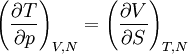
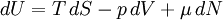
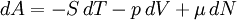
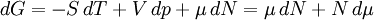
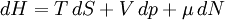
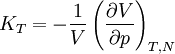
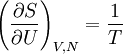
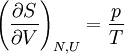
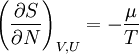
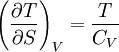
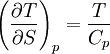
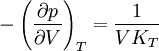
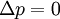
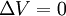
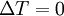
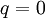
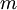
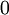
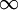
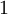
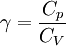
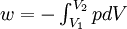
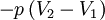
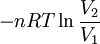
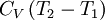
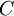
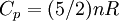
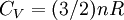
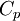 or
or 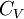
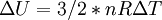
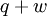
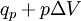
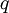
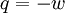
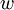
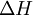
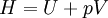
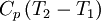
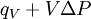
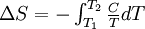
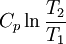
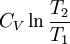
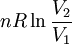
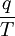
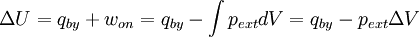
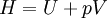
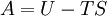
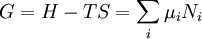
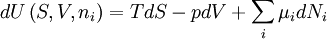
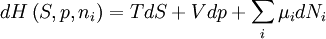
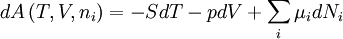
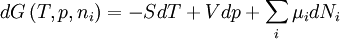
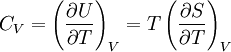
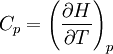
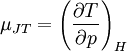
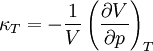
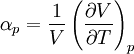
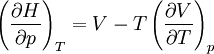
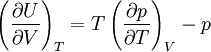
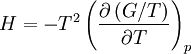
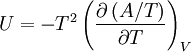
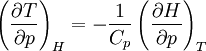
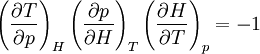
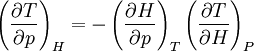
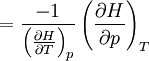 ;
;