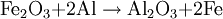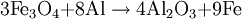To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Thermite
Thermite is a pyrotechnic composition of aluminium powder and a metal oxide which produces an aluminothermic reaction known as a thermite reaction. The aluminium is oxidized by the oxide of another metal, most commonly iron oxide (rust). The products are aluminium oxide, free elemental iron, and a large amount of heat. The reactants are commonly powdered and mixed with a binder to keep the material solid and prevent separation. The reaction is used for thermite welding, often used to join railroad rails. Some other metal oxides can be used, such as chromium oxide, to generate elementary metal. Copper thermite, using copper oxide, is used for creating electric joints in a process called cadwelding. Some thermite-like mixtures are used as pyrotechnic initiators such as fireworks. Additional recommended knowledge
HistoryThermite (Thermit) was discovered in 1893 and patented in 1895 by German chemist Dr. Hans Goldschmidt. Consequently, the reaction is sometimes called the "Goldschmidt reaction" or "Goldschmidt process". Dr. Goldschmidt was originally interested in producing very pure metals by avoiding the use of carbon in smelting, but he soon realized the value in welding. The first commercial application was the welding of tram tracks in Essen, in 1899. Degussa, a corporate descendant of Goldschmidt's firm, is still today one of the world's largest producers of welding thermite. TypesBlack or blue iron oxide (Fe3O4), produced by oxidizing iron in an oxygen-rich environment under high heat, is the most commonly used thermite oxidizing agent because it is inexpensive and easily produced. Red iron(III) oxide (Fe2O3, commonly known as rust) can also be used. Other oxides are occasionally used, such as MnO2 in manganese thermite, Cr2O3 in chromium thermite, or copper(II) oxide in copper thermite, but only for highly specialised purposes. All examples use aluminium as the reactive metal. Fluoropolymers can be used in special formulations; Teflon with magnesium or aluminium being a relatively common. Magnesium/teflon/viton is a pyrolant of this type. In principle, any reactive metal could be used instead of aluminium. This is rarely done, however, because the properties of aluminium are ideal for this reaction. It is by far the cheapest of the highly reactive metals; it also forms a passivation layer making it safer to handle than many other reactive metals. The melting and boiling points of aluminium also make it ideal for thermite reactions. Its relatively low melting point (660 °C, 1221 °F) means that it is easy to melt the metal, so that the reaction can occur mainly in the liquid phase[1] and thus proceeds fairly quickly. At the same time, its high boiling point (2519 °C, 4566 °F) enables the reaction to reach very high temperatures, since several processes tend to limit the maximum temperature to just below the boiling point.[2] Such a high boiling point is common among transition metals (e.g. iron and copper boil at 2887 °C and 2582 °C respectively), but is especially unusual among the highly reactive metals (cf. magnesium and sodium which boil at 1090 °C and 883 °C respectively). Although the reactants are stable at room temperature, they burn with an extremely intense exothermic reaction when they are heated to ignition temperature. The products emerge as liquids due to the high temperatures reached (up to 2500 °C (4500 °F) with iron(III) oxide)—although the actual temperature reached depends on how quickly heat can escape to the surrounding environment. Thermite contains its own supply of oxygen and does not require any external source of air. Consequently, it cannot be smothered and may ignite in any environment, given sufficient initial heat. It will burn well while wet and cannot be extinguished with water. Small amounts of water will boil before reaching the reaction. If thermite is ignited underwater, the molten iron produced will extract oxygen from water and generate hydrogen gas in a single-replacement reaction. This gas may, in turn, burn by combining with oxygen in the air. IgnitionConventional thermite reactions require very high temperatures for initiation. These cannot be reached with conventional black powder fuses, nitrocellulose rods, detonators, a suitable pyrotechnic initiator, or other common igniting substances. Even when the thermite is hot enough to glow bright red, it will not ignite as it must be at or near white-hot to initiate the reaction. It is possible to start the reaction using a propane torch if done correctly. The torch can preheat the entire pile of thermite which will make it explode instead of burning slowly when it finally reaches ignition temperature. Often, strips of magnesium metal are used as fuses. Because metals burn without releasing cooling gases, they can potentially burn at extremely high temperatures. Reactive metals such as magnesium can easily reach temperatures sufficiently high for thermite ignition. However, this method is notoriously unreliable: Magnesium itself is difficult to ignite, and in windy or wet conditions the strip may be extinguished. Also, magnesium strips do not contain their own source of oxygen so combustion cannot occur unless the magnesium strips are exposed to air. A significant danger of magnesium ignition is the fact that the metal is an excellent conductor of heat; heating one end of the ribbon may cause the other end to transfer enough heat to the thermite to cause premature ignition. Despite these issues, magnesium ignition remains popular amongst amateur thermite users, mainly because it can be easily obtained. The reaction between potassium permanganate and glycerine or ethylene glycol is used as an alternative to the magnesium method. When these two substances mix, a spontaneous reaction will begin, slowly increasing the temperature of the mixture until flames are produced. The heat released by the oxidation of glycerine is sufficient to initiate a thermite reaction. However, this method can also be unreliable and the delay between mixing and ignition can vary greatly due to factors such as particle size and ambient temperature. Apart from magnesium ignition, some amateurs also choose to use sparklers to ignite the thermite mixture. These reach the necessary temperatures and provide enough time before the burning point reaches the sample. However, this can be a dangerous method, as the iron sparks, like the magnesium strips, burn at thousands of degrees and can ignite the thermite even though the sparkler itself is not in contact with it. This is especially dangerous with finely powdered thermite. Similarly, finely-powdered thermite can be ignited by a regular flint spark lighter, as the sparks are burning metal (in this case, the highly-reactive rare-earth metals lanthanum and cerium). Therefore it is unsafe to strike a lighter close to thermite. A stoichiometric mixture of finely powdered iron(III) oxide and aluminium may be ignited using ordinary red-tipped book matches by partially embedding one match head in the mixture, and igniting that match head with another match, preferably held with tongs in gloves to prevent flash burns. Civilian usesThermite reactions have many uses. Thermite was originally used for repair welding in-place thick steel sections such as locomotive axle-frames where the repair can take place without removing the part from its installed location. It can also be used for quickly cutting or welding steel such as rail tracks, without requiring complex or heavy equipment. A thermite reaction, when used to purify the ores of some metals, is called the Thermite process, or aluminothermic reaction. An adaptation of the reaction, used to obtain pure uranium, was developed as part of the Manhattan Project at Ames Laboratory under the direction of Frank Spedding. It is sometimes called the Ames process. When thermite is made using iron (II) oxide, for maximum efficiency it should contain, by mass, 25.3% aluminium and 74.7% iron oxide. (This mixture is sold under the brand name Thermit as a heat source for welding.) The complete formula for the reaction using iron (III) oxide is as follows: ΔH = -851.5 kJ/mol[citation needed] When thermite is made using iron (II,III) oxide, for maximum efficiency it should contain, by mass, 23.7% aluminium and 76.3% iron oxide. The formula for the reaction using iron (II,III) oxide: ΔH = -3347.6 kJ/mol[citation needed] A modified version of this process (run under an inert atmosphere) can be used to produce a number of alloys—the mixture is usually ignited electrically. This approach has been used to prepare Ni-Al Alloys amongst others. Copper thermite is used for welding together thick copper wires for the purpose of electrical connections (cadwelding). Military usesThermite hand grenades are used as incendiary devices to quickly destroy enemy equipment. Additionally, thermite grenades are used by friendly forces to destroy their own items and equipment when there is imminent danger of capture. Because standard iron-thermite is difficult to ignite, burns with practically no flame and has a small radius of action, standard thermite is rarely used on its own as an incendiary composition. It is more usually employed with other ingredients added to enhance its incendiary effects. Thermate-TH3 is a mixture of thermite and pyrotechnic additives which have been found to be superior to standard thermite for incendiary purposes. Its composition by weight is generally 68.7% thermite, 29.0% barium nitrate, 2.0% sulfur and 0.3% binder. Addition of barium nitrate to thermite increases its thermal effect, creates flame in burning and significantly reduces the ignition temperature. Although the primary purpose of Thermate-TH3 is as an incendiary, it will also weld metal surfaces together. A classic military use for thermite is disabling artillery pieces and it has been used for this purpose since the Second World War. Thermite can permanently disable artillery pieces without the use of explosive charges and therefore can be used with a reasonable amount of stealth. There are several ways to do this. By far the most destructive method is to weld the weapon shut by inserting one or more armed thermite grenades into the breech and then quickly closing it. This makes the weapon impossible to load. An alternative method is to insert an armed thermite grenade down the muzzle of the artillery piece, fouling the barrel. This makes the piece very dangerous to fire. Yet another method is to use thermite to weld the traversing and elevation mechanism of the weapon, making it impossible to aim properly. Thermite was also used in both German and Allied incendiary bombs during WWII. Incendiary bombs usually consisted of dozens of thin thermite filled canisters (bomblets) ignited by a magnesium fuse. Incendiary bombs destroyed entire cities due to the raging fires that resulted from their use. Cities that were primarily consisted of wooden buildings were especially susceptible. These incendiary bombs were utilized primarily during night time air raids. Bomb sights could not be used at night, creating the need to use munitions that could destroy targets without the need for precision placement. HazardsThermite usage is hazardous due to the extremely high temperatures produced and the extreme difficulty in smothering a reaction once initiated. The thermite reaction releases dangerous ultra-violet (UV) light requiring that the reaction not be viewed directly, or that special eye protection (for example, a welder's mask) be worn. Small streams of molten iron released in the reaction can travel considerable distances and may melt through metal containers, igniting their contents. Additionally, flammable metals with relatively low boiling points such as zinc, whose boiling point of 907 °C (1665 °F) is about 1370 °C (2500 °F) below the temperature at which thermite burns, could potentially boil superheated metal violently into the air if near a thermite reaction, where it could then burst into flame as it is exposed to oxygen. Preheating of thermite before ignition can easily be done accidentally, for example by pouring a new pile of thermite over a hot, recently-ignited pile of thermite slag. When ignited, preheated thermite can burn almost instantaneously, releasing a much greater amount of light and heat energy than normal and causing burns and eye damage at what would normally be a reasonably safe distance. The thermite reaction can take place accidentally in industrial locations where abrasive grinding and cutting wheels are used with ferrous metals. Using aluminium in this situation produces an admixture of oxides which is capable of a violent explosive reaction.[3] Mixing water with thermite or pouring water onto burning thermite can cause a phreatomagmatic explosion, spraying hot fragments in all directions. See also
Notes
References
Categories: Inorganic reactions | Aluminium |
|||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Thermite". A list of authors is available in Wikipedia. |