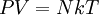To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Thermodynamic instrumentsA thermodynamic instrument is any device which facilitates the quantitative measurement of thermodynamic systems. In order for a thermodynamic parameter to be truly defined, a technique for its measurement must be specified. For example, the ultimate definition of temperature is "what a thermometer reads". The question follows - what is a thermometer? Additional recommended knowledgeLet's assume that we understand mechanics well enough to understand and measure volume, area, mass, and force. These may be combined to understand the concept of pressure, which is force per unit area and density, which is mass per unit volume. It has been experimentally determined that, at low enough pressures and densities, all gases behave as ideal gases. The behavior of an ideal gas is given by the ideal gas law: where P is pressure, V is volume, N is the number of particles (total mass divided by mass per particle), k is Boltzmann's constant, and T is temperature. In fact, this equation is more than a phenomenological equation, it gives an operational, or experimental, definition of temperature. A thermometer is a tool that measures temperature - a primitive thermometer would simply be a small container of an ideal gas, that was allowed to expand against atmospheric pressure. If we bring it into thermal contact with the system whose temperature we wish to measure, wait until it equilibrates, and then measure the volume of the thermometer, we will be able to calculate the temperature of the system in question via T=PV/Nk. Hopefully, the thermometer will be small enough that it does not appreciably alter the temperature of the system it is measuring, and also that the atmospheric pressure is not affected by the expansion of the thermometer. This discussion of the development of a thermometer introduces some general ideas about thermodynamic tools. Two general complementary tools are the meter and the reservoir.
The ideal gas thermometer can be defined more precisely by saying it is a system containing an ideal gas, which is thermally connected to the system it is measuring, while being dynamically and materially insulated from it. It is simultaneously dynamically connected to an external pressure reservoir, from which it is materially and thermally insulated. Other thermometers (e.g. mercury thermometers, which display the volume of mercury to the observer) may now be constructed, and calibrated against the ideal gas thermometer. Some common thermodynamic meters are:
Some common reservoirs are:
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Thermodynamic_instruments". A list of authors is available in Wikipedia. |