To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Yttrium barium copper oxide
Yttrium barium copper oxide, often abbreviated YBCO, is a chemical compound with the formula YBa2Cu3O7. This material, a famous "high-temperature superconductor", achieved prominence because it was the first material to achieve superconductivity above the boiling point of nitrogen. Additional recommended knowledge
HistorySeventy-five years after the discovery of superconductivity, Georg Bednorz and Alexander Müller, working at IBM in Zurich Switzerland, discovered that certain semiconducting oxides became superconducting at the then relatively high temperature of 35 K. In particular, the lanthanum barium copper oxides, an oxygen deficient perovskite-related material proved particularly promising. Building on the motif discovered by Bednorz and Müller, Maw-Kuen Wu and his graduate students, Ashburn and Torng[citation needed] at the University of Alabama in Huntsville in 1987 and Paul Chu and his students at the University of Houston in 1987 (see superconductor page for info), discovered YBCO. Their work led to a rapid succession of new high temperature superconducting materials, ushering in a new era in material science and chemistry. YBCO was the first material to become superconducting above 77 K, the boiling point of nitrogen. All materials developed before YBCO became superconducting only at temperatures near the boiling points of liquid helium or liquid hydrogen (Tb = 20.1 K). The significance of the discovery of YBCO is the breakthrough in the refrigerant used to cool the material to below the critical temperature. SynthesisYBCO was first synthesized by heating a mixture of the metal carbonates at temperatures between 1000 to 1300 K.[2][3]
Modern syntheses of YBCO use the corresponding oxides and nitrates.[3] The superconducting property of YBa2Cu3O{7-x} is sensitive to the value of x, its oxygen content. Only those materials with 0 ≤ x ≤ 0.5 are superconducting below Tc, and when x ~ 0 the material superconducts at the highest temperature, 95 K.[3] In addition to being sensitive to the stoichiometry of oxygen, the properties of YBCO are influenced by the crystallization methods. Care must be taken to sinter YBCO. YBCO is a crystalline material, and the best superconduction performance is obtained when crystal grain boundaries are aligned by careful control of annealing and quenching temperature rates. Numerous other methods to synthesize YBCO have developed since its discovery by Wu and his coworkers, such as chemical vapor deposition (CVD)[2][3], sol-gel[4], and aerosol[5] methods. These alternative methods, however, still require careful sintering to produce a quality product. StructureYBCO crystallises in a defect perovskite structure consisting of layers. The boundary of each layer is defined by planes of square planar CuO4 units sharing 4 vertices. The planes can some times be slightly puckered[2]. Perpendicular to these CuO2 planes are CuO4 ribbons sharing 2 vertices. The yttrium atoms are found between the CuO2 planes, while the barium atoms are found between the CuO4 ribbons and the CuO2 planes. This structural feature is illustrated in the figure below.
More detailsAlthough YBa2Cu3O7 is a well defined chemical compound with a specific structure and stoichiometry, materials with less than seven oxygen atoms per formula unit are non-stoichiometric compounds. The structure of these materials depends on the oxygen content. This nonstoichiometry is denoted by the ( δ) in the chemical formula. Withδ = 1 the O(1) sites in the Cu(1) layer are vacant and the structure is tetragonal. The tetragonal form of YBCO is insulating and does not superconduct. Increasing the oxygen content slightly causes more of the O(1) sites to become occupied. For δ< 0.65 Cu-O chains along the b-axis of the crystal are formed. Elongation of the b-axis changes the structure to orthorhombic, with lattice parameters of a = 3.82, b = 3.89, and c = 11.68 Å. Optimum superconducting properties occur when δ ~0.07 and all of the O(1) sites are occupied with few vacancies. In experiments where other elements are substituted at the Cu and Ba sites evidence has shown that conduction occurs in the Cu(2)O planes while the Cu(1)O(1) chains act as charge reservoirs, which provide carriers to the CuO planes. (cite needed!) However, this model fails to address superconductivity in the homologue Pr123 (Praseodymium instead of Yttrium)[6]. Furthermore the superconducting length scales show similar anisotropy, the penetration depth ( Applications in technologySeveral commercial applications of high temperature superconducting materials have been realized. For example, superconducting materials are finding use as magnets in magnetic resonance imaging, magnetic levitation, and Josephson junctions. YBCO has yet to be used in many applications involving superconductors for two primary reasons:
Finally, it should be noted that cooling materials to liquid nitrogen temperature is often not practical on a large scale, although many commercial magnets are routinely cooled to liquid helium temperatures. The most promising method developed to utilize this material involves deposition of YBCO on flexible metal tapes coated with buffering metal oxides. Texture can be introduced into the metal tape itself (the RABiTS process) or a textured ceramic buffer layer can be deposited, with the aid of an ion beam, on an untextured alloy substrate (the IBAD process). Subsequent oxide layers prevent diffusion of the metal from the tape into the superconductor while transferring the template for texturing the superconducting layer. Novel variants on CVD, PVD, and solution deposition techniques are used to produce long lengths of the final YBCO layer at high rates. Companies pursuing these processes include American Superconductor, Superpower (a division of Intermagnetics General Corp), Sumitomo, Fujikura, Nexans Superconductors, and European Advanced Superconductors. A much larger number of research institutes have also produced YBCO tape by these methods. Surface modification of YBCOSurface modification of materials has often led to new and improved properties. Corrosion inhibition, polymer adhesion and nucleation, preparation of organic superconductor/ insulator/high-Tc superconductor trilayer structures, and the fabrication of metal/insulator/ superconductor tunnel junctions have been developed using surface modified YBCO[7]. These molecular layered materials are synthesized using cyclic voltammetry. Thus far YBCO layered with alkylamines, arylamines, and thiols have been produced with varying stability of the molecular layer. It has been proposed that amines act as Lewis bases and bind to Lewis acidic Cu surface sites in YBa2Cu3O7 to form stable coordination bonds. Magnetic levitationSimilar to all superconductors, YBCO displays the Meissner effect as it is cooled and reaches its critical temperature. At the critical temperature and below, YBCO becomes perfectly diamagnetic and excludes all magnetic fields from passing through it by developing an internal magnetic field that perfectly balances the externally applied magnetic field. This internal field causes any magnet on the surface of the superconductor to levitate[2]. See full article Meissner Effect Media
References
Categories: Yttrium compounds | Barium compounds | Copper compounds | Oxides | High-temperature superconductors |
|||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Yttrium_barium_copper_oxide". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||





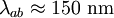 ,
,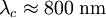 ) and the coherence length, (
) and the coherence length, (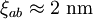 ,
,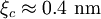 ). Although the coherence length in the a-b plane is 5 times greater than that along the c-axis it is quite small compared to classic superconductors such as niobium. (
). Although the coherence length in the a-b plane is 5 times greater than that along the c-axis it is quite small compared to classic superconductors such as niobium. (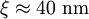 ). This modest coherence length means that the superconducting state is more susceptible to local disruptions from interfaces or defects on the order of a single unit cell, such as the boundary between twinned crystal domains. This sensitivity to small defects complicates fabricating devices with YBCO, and the material is also sensitive to degradation from humidity.
). This modest coherence length means that the superconducting state is more susceptible to local disruptions from interfaces or defects on the order of a single unit cell, such as the boundary between twinned crystal domains. This sensitivity to small defects complicates fabricating devices with YBCO, and the material is also sensitive to degradation from humidity.


