Nickel wraps itself in ultrathin carbon mantle
Metal offers improved method of making graphene
Scientists have been exploring a promising way of producing layers of graphene with particularly few defects. This coveted carbon allotrope spontaneously forms on nickel surfaces on which carbon has previously been deposited. The extremely high quality of the graphene produced and the relatively low temperature of around 400 degrees Celsius used in the process make this method interesting for practical applications, according to the researchers headed by Bernhard Klötzer from the University of Innsbruck. The team, which also includes scientists from the DESY NanoLab, is presenting its findings in the journal Scientific Reports.
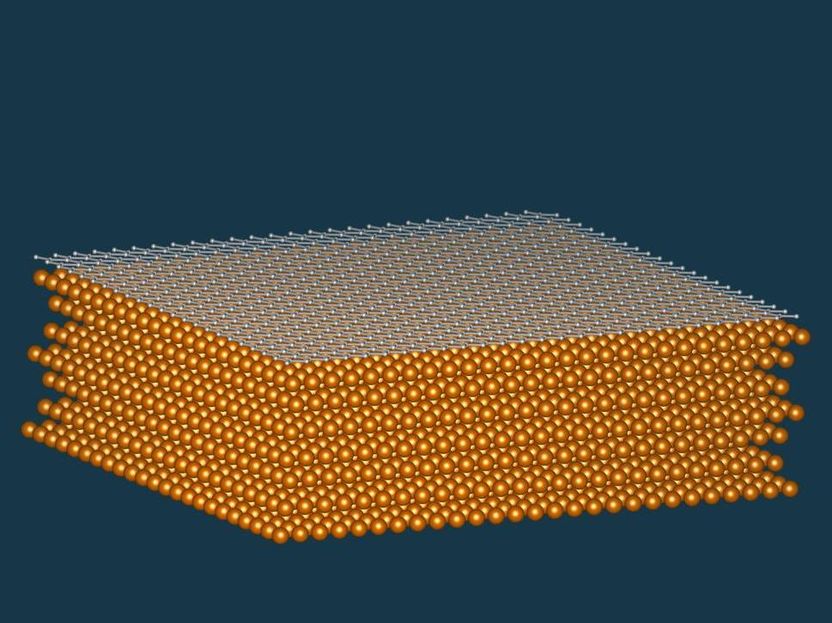
Nickel soaks up carbon like a sponge and forms high-quality graphene layers at its surface, due to the similar structure of the crystal lattices.
DESY, Vedran Vonk
Graphene consists of a single layer of carbon atoms which are arranged in an atomic array resembling chicken wire. The material exhibits an astonishing chemical and structural stability and a high electrical conductivity, making it particularly interesting for electronic applications, such as transistors, transparent electrodes, photovoltaic cells and batteries. On top of this, its special mechanical properties mean that it can be used to build filters and seals on a subnanometre scale. Being impermeable even to gases such as helium and water, graphene can for example serve as a transparent electrode or membrane. Used in the form of graphene oxide, on the other hand, the level of water diffusion can be precisely adjusted, a property than can be used for example in desalination applications. Numerous other unusual properties allow scientific experiments to be conducted that would not be possible using conventional materials. In 2010, the Dutch-British physicist Andre Geim and the Russian-British physicist Konstantin Novoselov were awarded the Nobel Prize for their ground-breaking work on graphene.
“In view of the many potential uses of graphene, there is a strong demand for optimised and cost-efficient ways of producing large sheets of it with few defects,” explains the head of research, Bernhard Klötzer. Today, the ultrathin carbon film is often made with the help of metals, to which the gas ethene is applied by vapour deposition. Ethene consists of two carbon and four hydrogen atoms (C2H4), which break apart when the temperature of the metal is high enough. The carbon is then deposited on the surface of the metal in the form of islands of graphene. However, the existing high-temperature methods often produce defects. These can take the form of individual carbon meshes being twisted with respect to the others, or holes within the atomic chicken wire, which mean that it is no longer gas-tight.
“Nickel lends itself to making graphene because its crystal lattice corresponds perfectly to the hexagonal graphene meshes,” explains co-author Vedran Vonk from the DESY NanoLab. “However, until now only tiny graphene regions with a diameter of a few micrometres had been observed on nickel.” This was in line with theoretical predictions, because in view of nickel’s crystal structure it was hitherto thought that the metal would not be able to adsorb enough carbon to produce an extensive layer of graphene. Although ethene also decomposes on a nickel surface, the metal bonds with the carbon as it is released, forming a so-called carbide on its surface. The carbon stored in this form can be released again by heating it gently. “However, about four times as much carbon is required for a continuous layer of graphene,” explains Vonk.
The researchers used high-intensity X-rays at the European Synchrotron Radiation Facility ESRF to examine the carbon-binding capacity of nickel more closely. They found that nickel not only stores carbon in gaps within its metal lattice and forms a carbide layer on its surface; in addition, a new carbide phase is formed beneath the surface of the metal. “The nickel soaks up the carbon like a sponge,” Vonk reports. The resulting carbon-supersaturated metal only needs to be heated slightly for a complete layer of graphene to spontaneously form on its surface.
“We were not expecting the amount of carbon in the pre-treated nickel to be sufficient to produce an uninterrupted layer of graphene,” says Klötzer. In fact, all three carbon reservoirs in the nickel are used up completely when the graphene forms. The scientists studied a nickel sample with a diameter of one centimetre, which was completely covered with a perfect layer of graphene within about 15 minutes. Since graphene formation normally starts independently at a number of different sites, the duration of this process does not depend much on the size of the surface.
“The relatively low temperature of around 400 degrees Celsius at which the process occurs could be an advantage, both for the quality of the graphene and for its industrial-scale production,” Klötzer emphasises. Nevertheless, some technical hurdles still need to be overcome before the technique can be applied. For example, a method needs to be found by which the graphene can be removed from the nickel without damaging it and without having to dissolve away too much of the metal – using an acid, for example. The substrate of choice for the potential industrial production of graphene therefore consists of ultrathin sheets of nickel with a perfect crystalline structure.
The University of Innsbruck, ESRF, the Fritz Haber Institute in Berlin, the University of Hamburg, the Technical University of Vienna and DESY were also involved in the research that has been conducted in the framework of the Austrian Collaborative Research Initiative Functional Oxide Surfaces and Interfaces that is funded by the Austrian Science Fund FWF.