Light makes pores bigger
Photo-growth of pores in a polymer gel network
Irradiation with light is an established method for initiating polymerization or crosslinking (curing) in the production of plastics. American researchers are now using light to retroactively increase the size of the pores within a polymer network. As reported in the journal Angewandte Chemie, this new approach allows for the production of polymer gels with tailored mechanical properties.
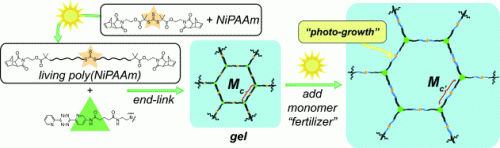
To achieve this, Huaxing Zhou and Jeremiah A. Johnson at the Massachusetts Institute of Technology had to incorporate photoreactive groups of atoms into a polymer. They chose to use trithiocarbonate units, which consist of one carbon atom and three sulfur atoms. The researchers attached organic side groups to two of the sulfur atoms. Exposure to UV light breaks a bond between the sulfur atom and the carbon atom of the side chain. This process forms highly reactive radicals (molecules with unpaired electrons) that want to form new bonds. If all of this takes place in the presence of N-isopropylacrylamide monomers, the monomers form a polymer. From a formal point of view, one monomer after another "pushes" itself in between the sulfur atom of the central thiocarbonate group and the side groups. The two side groups grow into long polymeric chains. As long as the irradiation continues, bond breaking and new bond formation can keep going on around the sulfur atoms. The reaction stops in the absence of light.
The researchers treat the resulting polymer with tris-tetrazine, which links together three of the polymer chains, causing them to crosslink into a gel. Even in this cross-linked state, the polymer chains are capable of continuing to grow outward from the trithiocarbonate groups if more monomer is added and irradiated. The longer the irradiation lasts, the longer the chains grow. This causes the size of the pores in the polymer network to increase. As a consequence, the gel's rigidity decreases, allowing it to swell further. They also showed that gel growth occurs under natural sunlight.
This process could be used to control a polymerization in both time and space. By using a mask, it should be possible to make a pattern of denser and looser regions or even a mechanical gradient within the gel. If the polymerization is carried out stepwise with different monomers, it should also be possible to make a chemical pattern or a chemical gradient, allowing for the controlled tuning of the size and composition of the pores within a gel.
This approach could be used to make new materials that physically grow towards a light source in order to optimize their properties. Applications could include light-harvesting materials, shape-defined filters for removal of toxic agents, scaffolds for studying how mechanical and chemical properties of materials interact with living cells/organisms, and new materials that "self-heal" after breaking.
Original publication
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.




























































