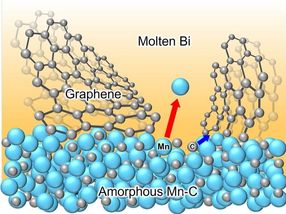
How to look for a few good catalysts
Two key physical phenomena take place at the surfaces of materials: catalysis and wetting. A catalyst enhances the rate of chemical reactions; wetting refers to how liquids spread across a surface.
Now researchers at MIT and other institutions have found that these two processes, which had been considered unrelated, are in fact closely linked.
"What's really exciting is that we've been able to connect atomic-level interactions of water and oxides on the surface to macroscopic measurements of wetting, whether a surface is hydrophobic or hydrophilic, and connect that directly with catalytic properties," says Yang Shao-Horn, the W.M. Keck Professor of Energy at MIT. The research focused on a class of oxides called perovskites that are of interest for applications such as gas sensing, water purification, batteries, and fuel cells.
Since determining a surface's wettability is "trivially easy," says senior author Kripa Varanasi, an associate professor of mechanical engineering, that determination can now be used to predict a material's suitability as a catalyst. Since researchers tend to specialize in either wettability or catalysis, this produces a framework for researchers in both fields to work together to advance understanding, says Varanasi, whose research focuses primarily on wettability; Shao-Horn is an expert on catalytic reactions.
Both effects are important in a variety of industrial processes and have been the subject of much empirical research
"It's primarily an experimental technique" that made the new understanding possible, explains Kelsey Stoerzinger, an MIT graduate student and the paper's lead author. While most attempts to study such surface science use instruments requiring a vacuum, this team used a device that could study the reactions in humid air, at room temperature, and with varying degrees of water vapor present. Experiments using this system, called ambient pressure X-ray photoelectron spectroscopy, revealed that the reactivity with water is key to the whole process, she says.
The water molecules break apart to form hydroxyl groups - bonded to the material's surface. These reactive compounds, in turn, are responsible for increasing the wetting properties of the surface, while simultaneously inhibiting its ability to catalyze chemical reactions. Therefore, for applications requiring high catalytic activity, the team found, a key requirement is that the surface be hydrophobic, or non-wetting.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.