Unveiling distribution of defects in proton conductors
Solid oxide fuel cells (SOFC), recently used as a power source for households in Japan, have several drawbacks such as high-cost, material degradation and long start-up time derived from high operating temperatures up to 750°C.
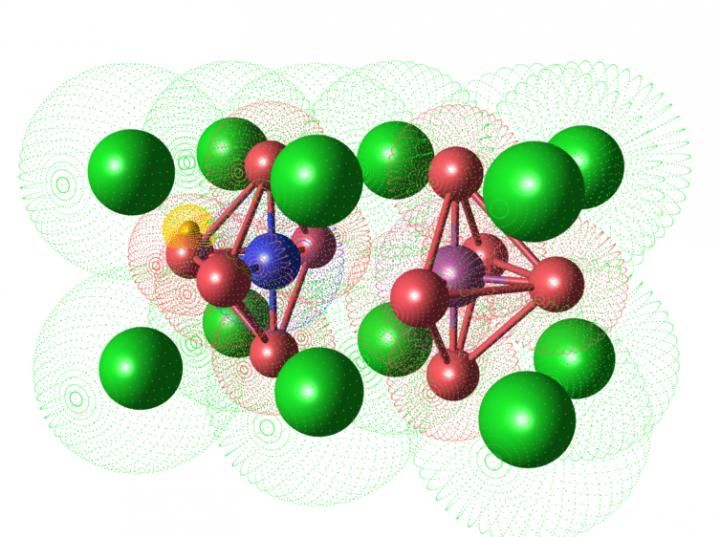
This figure shows the crystal structure of Sc-doped BaZrO3 to be used as an electrolyte material in intermediate temperature solid oxide fuel cells (IT-SOFCs). By using a nuclear magnetic resonance (NMR) technique, the distribution of proton and oxygen vacancy in Sc-doped BaZrO3 was clarified. Green, blue, purple and red spheres represent Ba, Zr, Sc cations and oxide ions in the Sc-doped BaZrO3, respectively. In this material, a proton (gold sphere) having one positive charge is known to be trapped around the Sc cation (purple sphere) since the Sc site possesses one negative charge; the trapped proton is the cause of low proton conductivity at the intermediate temperature range. Meanwhile, when an oxide ion (red sphere) is vacant (center position in this case), the pair of the vacancy and Sc now possesses one positive charge. The positive charge works to liberate the trapped proton from nearby Sc to nearby Zr (blue sphere). This change in proton distribution is believed to improve the proton conductivity of Sc-doped BaZrO3.
Hitoshi Takamura
Lowering the operating temperature to an "intermediate" range of 300-500°C would, in effect, enable the use of low-cost materials and allow for a quicker start-up which, in turn, could lead to wider commercial use and application to a mobile power source.
A team of researchers at Tohoku University in Japan has developed a new idea to improve proton conductivity in rare-earth doped BaZrO3 perovskite-type proton conductors. Rare-earth doped BaZrO3 is a promising candidate material for intermediate temperature SOFCs. However, further improvement of proton conductivity is required for practical use.
The researchers suggest a strategy to improve the mobility of protons by controlling oxygen vacancies as well as protons. Protons are known to be "trapped" around a rare-earth element in the doped BaZrO3 which lowers the proton conductivity. This proton trapping is originated from the electrostatic attractive interaction between a negatively charged rare-earth element and a positively charged proton.
However, when the pairing of a rare-earth element and an oxygen vacancy is created in the material, this pair possesses a positive net charge and therefore, inhibits the trapping of protons due to the electrostatic repulsive interaction.
In developing this idea, the team clarified the distribution of protons and oxygen vacancies in Sc-doped BaZrO3 by combining nuclear magnetic resonance spectroscopy and thermogravimetric analysis. When a certain amount of oxygen vacancies (4 mol%) exists in the material, the proton concentration around Zr is higher than that around the rare-earth element which indicates protons with less influence from the trapping effects of the rare-earth element.
"Because the attractive interaction between the rare-earth element and protons causes the proton trapping, introducing another defect having positive charges - that is to say, oxygen vacancy - appears to liberate the trapped protons," said Hitoshi Takamura who led the research at Tohoku University. He and his colleagues have clarified that the interaction between the rare-earth element and oxygen vacancy does prevent the proton trapping.
"This idea can be applied not only to the development of ionic conductors but also other materials, such as fluorescent and catalyst materials, since the interaction of defects plays an important role in these materials," said Takamura. "If the distribution of defects becomes controllable, we can design a variety of functional materials. That is our goal for this research."
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!


























































