Neutron diffraction studies reveal origins of deterioration in lithium batteries
Researchers use diffraction data from pulsed high-intensity neutrons to understand the reactions that deteriorate lithium batteries during operation.
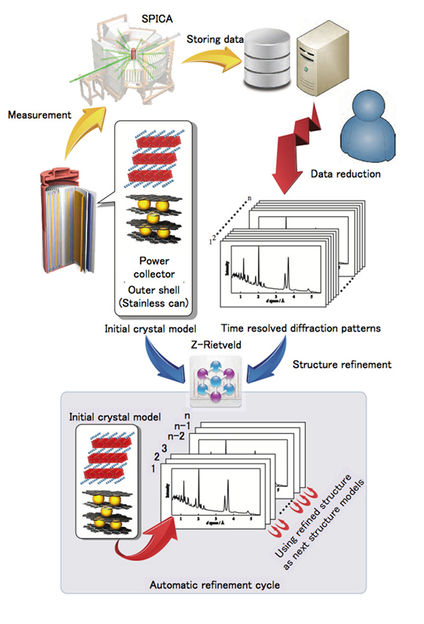
Top left: schematic diagram and cell environment for the in situ experiment using an 18,650 cell in the SPICA diffractometer, with examples of the data analysis using the data analysis program developed in the present study. A schematic diagram shows the flow chart of the analysis process for the automatic refinement cycle.
Tokyo Institute of Technology
Despite the commercialisation of rechargeable lithium batteries in 1991, improvements to the reliability, safety, and long-term stability are still needed for their use in a wider range of large-scale applications. Now a collaboration of researchers in Japan have developed a technique they can use while the battery is in operation, which may shed light on some of the reactions that occur in these systems, providing insights for better battery design.
While techniques exist to monitor reaction dynamics, so far they have been difficult to apply on commercial batteries because of the stainless steel cans around them and their size. Ryoji Kanno worked with researchers at Tokyo Institute of Technology, High Energy Accelerator Research Organization (KEK), Sokendai (the Graduate University for Advanced Studies), and Kyoto University. They developed an in operando diffraction technique that uses pulsed high-intensity neutrons and a high-resolution high-intensity time-of-flight diffractometer to monitor the processes in the battery while it is in action.
The high current that results from pulsed operation causes inhomogeneous reactions and a lithium concentration gradient, so that relaxation processes then ensue once the current is turned off. With their approach Kanno and colleagues were able to detect this relaxation process in a commercial cell under high current drain, for the first time. They also noted structural changes that indicate differences between charging and discharging, and suggest the first observation of the lithium composition ranges used for the reactions at the electrodes shifting with the cycle rate.
They also point out that while balancing lithium concentrations between cathode and anode is key to battery design, the non-equilibrium reaction conditions may change these concentrations. Such changes are strongly correlated with the formation of a “solid electrolyte interphase”, and the deterioration of the electrodes. They conclude, “This information provides basic battery reaction data to clarify the deterioration mechanism during cycling and under high-temperature operation and storage conditions.”
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.