A little tension yields enormous solar crystals
In the race to replace silicon in low-cost solar cells, semiconductors known as metal halide perovskites are favored because they can be solution-processed into thin films with excellent photovoltaic efficiency. A collaboration between KAUST and Oxford University researchers has now uncovered a strategy that grows perovskites into centimeter-scale, highly pure Crystals thanks to the effect of surface tension.
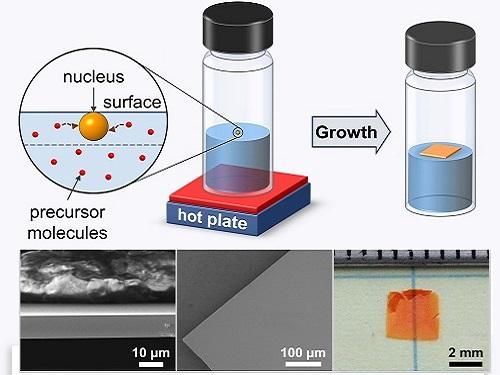
In-depth analysis of the mechanisms that generate floating crystals from hot liquids could lead to large-scale, printable solar cells
Reproduced with permission from reference 1© 2017 American Chemical Society
In their natural state, perovskites have difficultly moving solar-generated electricity because they crystallize with randomly oriented grains. Osman Bakr from KAUST's Solar Center and coworkers are working on ways to dramatically speed up the flow of these charge carriers using inverse temperature crystallization (ITC). This technique uses special organic liquids and thermal energy to force perovskites to solidify into structures resembling single crystals--the optimal arrangements for device purposes.
While ITC produces high-quality perovskites far faster than conventional chemical methods, the curious mechanisms that initiate crystallization in hot organic liquids are poorly understood. Ayan Zhumekenov, a PhD student in Bakr's group, recalls spotting a key piece of evidence during efforts to adapt ITC toward large-scale manufacturing. "At some point, we realized that when crystals appeared, it was usually at the solution's surface," he says. "And this was particularly true when we used concentrated solutions."
The KAUST team partnered with Oxford theoreticians to identify how interfaces influence perovskite growth in ITC. They propose that metal halides and solvent molecules initially cling together in tight complexes that begin to stretch and weaken at higher temperatures. With sufficient thermal energy, the complex breaks and perovskites begin to crystallize.
But interestingly, the researchers found that complexes located at the solution surface can experience additional forces due to surface tension--the strong cohesive forces that enable certain insects to stride over lakes and ponds. The extra pull provided by the surface makes it much easier to separate the solvent-perovskite complexes and nucleate crystals that float on top of the liquid.
Exploiting this knowledge helped the team produce centimeter-sized, ultrathin single crystals and prototype a photodetector with characteristics comparable to state-of-the-art devices. Although the single crystals are currently fragile and difficult to handle due to their microscale thicknesses, Zhumekenov explains that this method could help direct the perovskite growth onto specific substrates.
"Taking into account the roles of interfaces and surface tension could have a fundamental impact," he says, "we can get large-area growth, and it's not limited to specific metal cations--you could have a library of materials with perovskite structures."
Original publication
Most read news
Original publication
Ayan A. Zhumekenov et al.; "The Role of Surface Tension in the Crystallization of Metal Halide Perovskites"; ACS Energy Letters; 2017
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.





























































