Missing atoms in a forgotten crystal bring luminescence
A little-studied member of the perovskite family of materials could find use in a range of electronic devices, after researchers at KAUST discovered the secret of its strong photoluminescence.

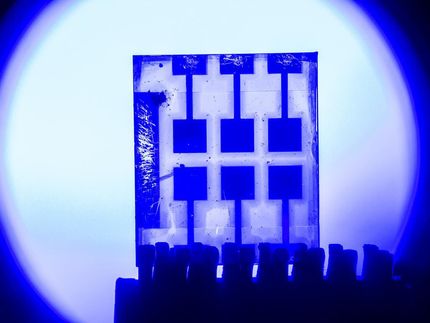
The perovskite has a strong green fluorescence.
© 2017 De Bastiani
Perovskites are a wide group of materials that are known to have remarkable optical and electronic properties. Perovskites with the general formula ABX3, and particularly the perovskite methylammonium lead trihalide, have attracted almost all the research attention thanks to their great promise as low-cost, high-efficiency solar cell materials.
Other members of the perovskite family and perovskite derivatives are also worthy research subjects, says Michele De Bastiani, a postdoctoral researcher in Osman Bakr's group at KAUST.
De Bastiani and his colleagues have been testing Cs4PbBr6, a perovskite of the A4BX6 branch of the family. This material is noted for its strong photoluminescence--the ability to absorb light at one wavelength and re-emit it at another.
The material's potential applications include color-converting coatings on LED light bulbs, lasers and photodetectors. But to be able to fine-tune the material's optoelectric properties for each application, researchers need to solve the mystery of why the perovskite photoluminesces so strongly.
"We investigated the structural and optoelectronic properties of Cs4PbBr6 to understand the origin of its photoluminescence," De Bastiani says. Subjecting the material to a barrage of tests, the team discovered that when a Cs4PbBr6 crystal was heated to 180°C, its photoluminescence was irreversibly destroyed.
Photoluminescence is a two-step process; absorption of light generates a pair of quasi-particles called excitons within the perovskite, which must recombine to re-emit the light. Using temperature-dependent X-ray diffraction to track structural changes to the material as heat was applied, the team discovered that at 180°C, CsPbBr3 nanocrystals form within the mineral.
The heat-induced structural rearrangements that create these nanocrystals also swallow natural defects in the original crystal where bromine atoms were missing, the researchers concluded. These bromine vacancies act as traps for passing exictons. Confined in these traps, the excitons are much more likely to recombine and emit light.
"Now that we have this fundamental understanding, our next step is to move on to potential applications," De Bastiani says. "The unique photoluminescence manifested by Cs4PbBr6 makes these perovskites compelling materials for electroluminescence devices, lasers and light converters."
Meanwhile, many other little-explored members of the perovskite family with interesting properties are waiting to be revealed, De Bastiani adds. "One example is CsPb2Br5, a single crystal we recently synthesized for the first time with unseen optoelectronic properties."
Original publication
Michele De Bastiani, Ibrahim Dursun, Yuhai Zhang, Buthainah A. Alshankiti, Xiao-He Miao, Jun Yin, Emre Yengel, Erkki Alarousu, Bekir Turedi, Jawaher M. Almutlaq, Makhsud I. Saidaminov, Somak Mitra, Issam Gereige, Ahmed AlSaggaf, Yihan Zhu, Yu Han, Iman S. Roqan, Jean-Luc Bredas, Omar F. Mohammed, and Osman M. Bakr; "Inside Perovskites: Quantum Luminescence from Bulk Cs4PbBr6 Single Crystals"; Chemistry of Materials; 2017
Most read news
Original publication
Michele De Bastiani, Ibrahim Dursun, Yuhai Zhang, Buthainah A. Alshankiti, Xiao-He Miao, Jun Yin, Emre Yengel, Erkki Alarousu, Bekir Turedi, Jawaher M. Almutlaq, Makhsud I. Saidaminov, Somak Mitra, Issam Gereige, Ahmed AlSaggaf, Yihan Zhu, Yu Han, Iman S. Roqan, Jean-Luc Bredas, Omar F. Mohammed, and Osman M. Bakr; "Inside Perovskites: Quantum Luminescence from Bulk Cs4PbBr6 Single Crystals"; Chemistry of Materials; 2017
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.