New X-ray spectroscopy explores hydrogen-generating catalyst
Using a newly developed technique, researchers from Japan, Germany and the U.S. have identified a key step in production of hydrogen gas by a bacterial enzyme. Understanding these reactions could be important in developing a clean-fuel economy powered by hydrogen.
Using a newly developed technique and one of the world's most advanced X-ray sources, researchers from Japan, Germany and the US are studying enzymes that can produce hydrogen gas. Understanding these reactions could be important in developing a clean-fuel economy powered by hydrogen.
Cramer Lab, UC Davis
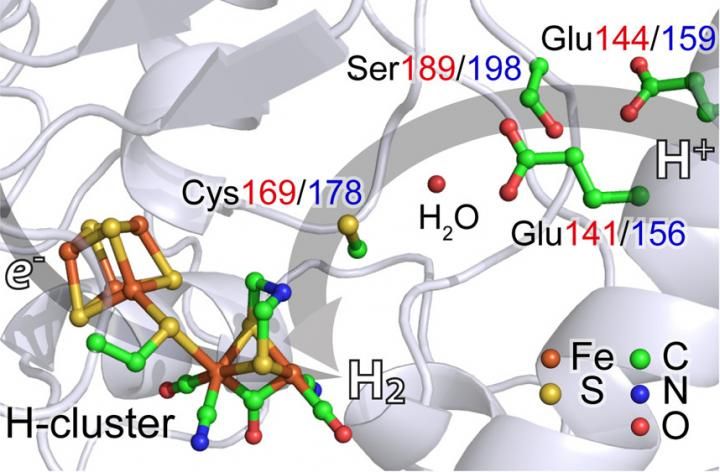
The team studied hydrogenases - enzymes that catalyze production of hydrogen from two widely distributed organisms: Chlamydomonas reinhardtii, a single-cell algae and Desulfovibrio desulfuricans, a bacterium.
In both cases, their hydrogenase enzymes have an active site with two iron atoms.
"Among hydrogenases, [FeFe] hydrogenase has the highest turnover rate (molecular hydrogen production rate) and therefore has a potential role in the future hydrogen economy, either by a direct use or by a synthetic complex which has a similar reaction center," said Professor Stephen P. Cramer in the UC Davis Department of Chemistry and coauthor on the paper along with graduate students Cindy C. Pham (co-first author) and Nakul Mishra and project scientist Hongxin Wang in the same department.
The researchers used a technique called nuclear resonant vibrational spectroscopy (NRVS) to follow the vibrational structures and analysis activity at the iron atoms in the enzyme. NRVS requires special equipment, and is currently only available at four sites in the world: the SPring-8 synchrotron in Hyogo, Japan, where this study was carried out; the Advanced Photon Source at Argonne National Laboratory, Illinois; the European Synchrotron Radiation Facility in Grenoble, France; and Petra-III in Hamburg, Germany.
Using NRVS, the team could show that the iron atoms briefly form a hydride (iron-hydrogen) before releasing molecular hydrogen (H2). It's the first successful experiment of its type on naturally occurring [FeFe] hydrogenases, Wang said.
"The successful outcome of this research is due to the broad collaboration between biochemists, spectroscopists, experimental physicists and theoreticians," Wang said. "This starts a journey to pursue iron specific information for all the intermediates in [FeFe] hydrogenase in the future."
Original publication
Vladimir Pelmenschikov, James A. Birrell, Cindy C. Pham, Nakul Mishra, Hongxin Wang, Constanze Sommer, Edward Reijerse, Casseday P. Richers, Kenji Tamasaku, Yoshitaka Yoda, Thomas B. Rauchfuss, Wolfgang Lubitz, and Stephen P. Cramer; "Reaction Coordinate Leading to H2 Production in [FeFe]-Hydrogenase Identified by Nuclear Resonance Vibrational Spectroscopy and Density Functional Theory"; JACS; 2017
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!