To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Creep (deformation)
Creep is the term used to describe the tendency of a material to move or to deform permanently to relieve stresses. Material deformation occurs as a result of long term exposure to levels of stress (physics) that are below the yield strength or ultimate strength of the material. Creep is more severe in materials that are subjected to heat for long periods and near melting point. Creep is often observed in glasses. Creep is a monotonically increasing function of temperature. The rate of this deformation is a function of the material properties, exposure time, exposure temperature and the applied load (stress). Depending on the magnitude of the applied stress and its duration, the deformation may become so large that a component can no longer perform its function — for example creep of a turbine blade will cause the blade to contact the casing, resulting in the failure of the blade. Creep is usually of concern to engineers and metallurgists when evaluating components that operate under high stresses or high temperatures. Creep is not necessarily a failure mode, but is instead a deformation mechanism. Moderate creep in concrete is sometimes welcomed because it relieves tensile stresses that otherwise may have led to cracking.
The temperature range in which creep deformation may occur differs in various materials. For example, Tungsten requires a temperature in the thousands of degrees before creep deformation can occur while ice formations such as the Antarctic ice cap will creep in freezing temperatures. Generally, the minimum temperature required for creep deformation to occur is 30-40% of the melting point for metals and 40-50% of melting point for ceramics. Virtually any material will creep upon approaching its melting temperature. Since the minimum temperature is relative to melting point, creep can be seen at relatively low temperatures for some materials. Plastics and low-melting-temperature metals, including many solders, creep at room temperature as can be seen marked in old lead hot-water pipes. Planetary ice is often at a high temperature relative to its melting point, and creeps. Creep deformation is important not only in systems where high temperatures are endured such as nuclear power plants, jet engines and heat exchangers, but also in the design of many everyday objects. For example, metal paper clips are stronger than plastic ones because plastics creep at room temperatures. Aging glass windows are often erroneously used as an example of this phenomenon: creep would only occur at temperatures above the glass transition temperature around 900°F/500°C. An example of an application involving creep deformation is the design of tungsten lightbulb filaments. Sagging of the filament coil between its supports increases with time due to creep deformation caused by the weight of the filament itself. If too much deformation occurs, the adjacent turns of the coil touch one another, causing an electrical short and local overheating, which quickly leads to failure of the filament. The coil geometry and supports are therefore designed to limit the stresses caused by the weight of the filament, and a special tungsten alloy with small amounts of oxygen trapped in the crystallite grain boundaries is used to slow the rate of coble creep. In steam turbine power plants, steam pipes carry superheated vapor under high temperature (1050°F/565.5°C) and high pressure often at 3500 psiMPa or greater. In a jet engine temperatures may reach to 1000°C, which may initiate creep deformation in a weak zone. For these reasons, it is crucial for public and operational safety to understand creep deformation behavior of engineering materials. Additional recommended knowledge
Stages of creepIn the initial stage, known as primary creep, the strain rate is relatively high, but slows with increasing strain. The strain rate eventually reaches a minimum and becomes near-constant. This is known as secondary or steady-state creep. This stage is the most understood. The characterized "creep strain rate", typically refers to the rate in this secondary stage. The stress dependence of this rate depends on the creep mechanism. In tertiary creep, the strain-rate exponentially increases with strain. Mechanisms of creepThe mechanism of creep depends on temperature and stress. The various methods are:
The most common mechanism is climb-assisted glide. General creep equation
where ε is the creep strain, C is a constant dependent on the material and the particular creep mechanism, m and b are exponents dependent on the creep mechanism, Q is the activation energy of the creep mechanism, σ is the applied stress, d is the grain size of the material, k is Boltzmann's constant, and T is the absolute temperature. Dislocation creepAt high stresses (relative to the shear modulus), creep is controlled by the movement of dislocations. When a stress is applied to a material, plastic deformation occurs due to the movement of dislocations in the slip plane. Materials contain a variety of defects, for example solute atoms, that act as obstacles to dislocation motion. Creep arises from this because of the phenomenon of dislocation climb. At high temperatures vacancies in the crystal can diffuse to the location of a dislocation and cause the dislocation to move to an adjacent slip plane. By climbing to adjacent slip planes dislocations can get around obstacles to their motion, allowing further deformation to occur. Because it takes time for vacancies to diffuse to the location of a dislocation this results in time dependent strain, or creep. For dislocation creep Q = Qself diffusion, m = 4-6, and b=0. Therefore dislocation creep has a strong dependence on the applied stress and no grain size dependence. Some alloys exhibit a very large stress exponent (n > 10), and this has typically been explained by introducing a "threshold stress," σth, below which creep can't be measured. The modified power law equation then becomes:
Nabarro-Herring CreepNabarro-Herring creep is a form of diffusion controlled creep. In N-H creep atoms diffuse through the lattice causing grains to elongate along the stress axis. For Nabarro-Herring creep k is related to the diffusion coefficient of atoms through the lattice, Q = Qself diffusion, m=1, and b=2. Therefore N-H creep has a weak stress dependence and a moderate grain size dependence, with the creep rate decreasing as grain size is increased. Nabarro-Herring creep is found to be strongly temperature dependent. For lattice diffusion of atoms to occur in a material, neighboring lattice sites or interstitial sites in the crystal structure must be free. A given atom must also overcome the energy barrier to move from its current site (it lies in an energetically favorable potential well) to the nearby vacant site (another potential well). The general form of the diffusion equation is D = DoExp(Ea / KT) where Do has a dependence on both the attempted jump frequency and the number of nearest neighbor sites and the probability of the sites being vacant. Thus there is a double dependence upon temperature. At higher temperatures the diffusivity increases due to the direct temperature dependence of the equation, the increase in vacancies through Schottky defect formation, and an increase in the average energy of atoms in the material. Nabarro-Herring creep dominates at very high temperatures relative to a material's melting temperature. Coble CreepCoble creep is a second form of diffusion controlled creep. In Coble creep the atoms diffuse along grain boundaries to elongate the grains along the stress axis. This causes Coble creep to have a stronger grain size dependence than N-H creep. For Coble creep k is related to the diffusion coefficient of atoms along the grain boundary, Q = Qgrain boundary diffusion, m=1, and b=3. Because Qgrain boundary diffusion < Qself diffusion, Coble creep occurs at lower temperatures than N-H creep. Coble creep is still temperature dependent, as the temperature increases so does the grain boundary diffusion. However, since the number of nearest neighbors is effectively limited along the interface of the grains, and thermal generation of vacancies along the boundaries is less prevalent, the temperature dependence is not as strong as in Nabarro-Herring creep. It also exhibits the same linear dependence on stress as N-H creep. Creep of PolymersCreep can occur in polymers and metals which are considered viscoelastic materials. When a polymeric material is subjected to an abrupt force, the response can be modeled using the Kelvin-Voigt Model. In this model, the material is represented by a Hookean spring and a Newtonian dashpot in parallel. The creep strain is given by: Where:
When subjected to a step constant stress, viscoelastic materials experience a time-dependent increase in strain. This phenomenon is known as viscoelastic creep. At a time t0, a viscoelastic material is loaded with a constant stress that is maintained for a sufficiently long time period. The material responds to the stress with a strain that increases until the material ultimately fails. When the stress is maintained for a shorter time period, the material undergoes an initial strain until a time t1, after which the strain immediately decreases (discontinuity) then gradually decreases at times t > t1 to a residual strain. Viscoelastic creep data can be presented in one of two ways. Total strain can be plotted as a function of time for a given temperature or temperatures. Below a critical value of applied stress, a material may exhibit linear viscoelasticity. Above this critical stress, the creep rate grows disproportionately faster. The second way of graphically presenting viscoelastic creep in a material is by plotting the creep modulus (constant applied stress divided by total strain at a particular time) as a function of time.[1] Below its critical stress, the viscoelastic creep modulus is independent of stress applied. A family of curves describing strain versus time response to various applied stress may be represented by a single viscoelastic creep modulus versus time curve if the applied stresses are below the material's critical stress value. Additionally, the molecular weight of the polymer of interest is known to affect its creep behavior. The effect of increasing molecular weight tends to promote secondary bonding between polymer chains and thus make the polymer more creep resistant. Similarly, aromatic polymers are even more creep resistant due to the added stiffness from the rings. Both molecular weight and aromatic rings add to polymers' thermal stability, increasing the creep resistance of a polymer. (Meyers and Chawla, 1999, 573) Both polymers and metals can creep.[2] Polymers experience significant creep at all temperatures above ~-200°C, however there are three main differences between polymetric and metallic creep. Metallic creep:[2]
Other examples
See also
References
|
||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Creep_(deformation)". A list of authors is available in Wikipedia. | ||||||||||||||





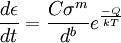
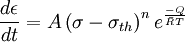 where
where 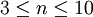 ).
).
![\epsilon(t) = \sigma C_0 + \sigma C \int_0^\infty f(\tau)(1-\exp[-t/ \tau]) d \tau](images/math/3/a/6/3a6cb5ddea04b9caf1a799d4b82b7447.png)


