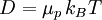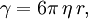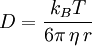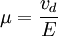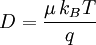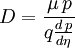To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Einstein relation (kinetic theory)In physics (namely, in kinetic theory) the Einstein relation is a previously unexpected connection revealed by Einstein in his 1905 paper on Brownian motion: Additional recommended knowledgelinking D, the Diffusion constant, and μp, the mobility of the particles; where kB is Boltzmann's constant, and T is the absolute temperature. The mobility μp is the ratio of the particle's terminal drift velocity to an applied force, μp = vd / F. This equation is an early example of a fluctuation-dissipation relation. Diffusion of particlesIn the limit of low Reynolds number, the mobility μ is the inverse of the drag coefficient γ. For spherical particles of radius r, Stokes' law gives where η is the viscosity of the medium. Thus the Einstein relation becomes This equation is also known as the Stokes-Einstein Relation. We can use this to estimate the Diffusion coefficient of a globular protein in aqueous solution: For a 100 kDalton protein, we obtain D ~10-10 m² s-1, assuming a "standard" protein density of ~1.2 103 kg m-3. Electrical conductionWhen applied to electrical conduction, it is normal to divide through by the charge q of the charge carriers, defining electron mobility (or hole mobility) where E is the applied electric field; so the Einstein relation becomes In a semiconductor with an arbitrary density of states the Einstein relation is where η is the chemical potential and p the particle number |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Einstein_relation_(kinetic_theory)". A list of authors is available in Wikipedia. |