To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Kröger-Vink NotationKröger-Vink Notation is set of conventions used to describe electrical charge and lattice position for defect species in crystals. It was proposed by F.A. Kröger and H.J. Vink. Additional recommended knowledgeGeneral format
M corresponds to the species. These would include:
S indicates the lattice site that the species occupies. For instance, Ni might occupy a Cu site. In this case, M would be replaced by Ni and S would be replaced by Cu. The site may also be a lattice interstice. In this case the symbol 'i' is used. C corresponds to the electric charge of the species relative to the site that it occupies. To continue the previous example, Ni often has the same valency as Cu, so the relative charge is zero. To indicate null charge, Examples
Categories: Chemical properties | Crystallography |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Kröger-Vink_Notation". A list of authors is available in Wikipedia. |





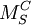
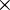 is used. A single
is used. A single  indicates a single positive charge, while two would represent two positive charges. Finally, ' signifies a single negative charge, so two, would indicate a double negative charge.
indicates a single positive charge, while two would represent two positive charges. Finally, ' signifies a single negative charge, so two, would indicate a double negative charge.
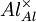 = an aluminium ion sitting on an aluminium lattice site, with neutral charge.
= an aluminium ion sitting on an aluminium lattice site, with neutral charge.
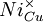 = a nickel ion sitting on a copper lattice site, with neutral charge.
= a nickel ion sitting on a copper lattice site, with neutral charge.
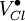 = a chlorine vacancy, with singular positive charge.
= a chlorine vacancy, with singular positive charge.
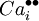 = a calcium interstitial ion, with double positive charge.
= a calcium interstitial ion, with double positive charge.
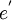 = an electron. A site isn't normally specified.
= an electron. A site isn't normally specified.


