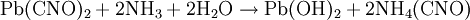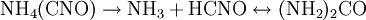To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Wöhler synthesisThe Wöhler synthesis is the conversion of ammonium cyanate into urea . This chemical reaction was discovered in 1828 by Friedrich Wöhler in an attempt to synthesize ammonium cyanate itself and is considered the starting point of modern organic chemistry. Although the Wöhler reaction concerns the conversion of ammonium cyanate, this salt only appears as an (unstable) intermediate. Wöhler demonstrated the reaction in his original publication with different sets of reactants: a combination of cyanic acid and ammonia, a combination of silver cyanate and ammonium chloride, a combination of lead cyanate and ammonia and finally from a combination of mercury cyanate and cyanatic ammonia (which is again cyanic acid with ammonia) . Additional recommended knowledgeIn a practical demonstration of the reaction the starting materials are a solution of potassium cyanate and ammonium chloride. The solutions are mixed, heated and cooled again. An additional proof of the chemical transformation is obtained by adding a solution of oxalic acid which forms urea oxalate as a white precipitate . Alternatively the reaction can be carried out with lead cyanate and ammonia . The actual reaction taking place is a double displacement reaction to form ammonium cyanate: Ammonium cyanate decomposes to ammonia and cyanic acid which in turn react to urea in a nucleophilic addition followed by tautomeric isomerization: Complexation with oxalic acid helps drive this chemical equilibrium to completion. The Wöhler synthesis is of great historical significance because for the first time an organic compound was produced from inorganic reactants. This finding went against the mainstream theory of that time called Vitalism which stated that organic matter possessed a special force or vital force inherent to all things living. For this reason a sharp boundary existed between organic and inorganic compounds. Urea was discoved in 1799 and could until then only be obtained from biological sources such as urine. Wöhler reported to his mentor Berzelius "I cannot, so to say, hold my chemical water and must tell you that I can make urea without thereby needing to have kidneys, or anyhow, an animal, be it human or dog". It is argued that organic chemistry did not actually start with this discovery in 1828 but 4 years earlier with the synthesis of acetic acid in 1824 also by Wöhler and also from the inorganic precursor cyanic acid. It is also argued that Vitalism was not put to bed either in 1828. His contemparies Liebig and Pasteur never abandoned Vitalism and it took until 1845 when Kolbe repeated an inorganic - organic conversion of carbon disulfide to acetic acid before Vitalism started to lose supporters in serious numbers. References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Wöhler_synthesis". A list of authors is available in Wikipedia. |