To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Critical radius
Critical radius is the minimum size that must be formed by atoms clustering together in the liquid before the solid particle is stable and begins to grow. Additional recommended knowledgeThe atoms would then form a dendrite. The crystal growth continues in three dimensions, the atoms attaching themselves in certain preferred directions, usually along the axes of a crystal, forming a characteristic tree-like structure of a dendrite. example: the critical radius for spheric-like dendride in an ideal system is
where γ is the surface energy, and δGv is Gibbs energy per volume. See alsoReferences
|
||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Critical_radius". A list of authors is available in Wikipedia. |





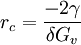 ,
,


