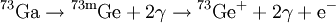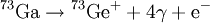To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Delayed nuclear radiationDelayed nuclear radiation can occur in a nuclear decay. It happens when an isotope decays into a very short-lived isotope and then decays again to a relatively long-lived isotope. The short-lived isotope is usually a meta-stable nuclear isomer. Additional recommended knowledgeFor example, Gallium-73 decays via beta decay into Germanium-73m which is very short-lived. The Germanium isotope emits two weak gamma rays and a conversion electron. Because the middle isotope is so short-lived, the gamma rays are considered part of the Gallium decay. Therefore the above equation is simplified. However, since there is a short time delay between the beta decay and the high energy gamma emissions and the third and fourth gamma rays, it is said that the lower energy gamma rays are delayed. Delayed gamma emissions are the most common form of delayed radiation but it is not the only form. It is common for the short-lived isotopes to have delayed emissions of various particles. In these cases it is commonly called a beta-delayed emission. This is because the decay is delayed until a beta decay takes place. For instance nitrogen-17 emits two beta-delayed neutrons after its primary beta emission. Just as in the above delayed gamma emission, the nitrogen is not the actual source of the neutrons, a short lived isotope of oxygen is. See also
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Delayed_nuclear_radiation". A list of authors is available in Wikipedia. |