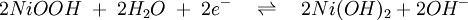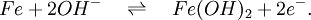To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Nickel-iron battery
The nickel-iron battery is a storage battery having a nickel(III) oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide. The active materials are held in nickel-plated steel tubes or perforated pockets. The nominal cell voltage is 1.2V. It is a very robust battery which is tolerant of abuse, (overcharge, overdischarge, short-circuiting and thermal shock) and can have very long life even if so treated. It is often used in backup situations where it can be continuously charged and can last for more than 20 years[citation needed]. Its limitations, namely, low specific energy, poor charge retention, and poor low-temperature performance, and its high cost of manufacture compared with the lead-acid battery led to a decline in usage. [1] Additional recommended knowledgeThe ability of these batteries to survive frequent cycling is due to the low solubility of the reactants in the electrolyte. The formation of metallic iron during charge is slow because of the low solubility of the Fe3O4, which is good and bad. It is good because the slow formation of iron crystals preserves the electrodes, bad because it limits the high rate performance: these cells charge slowly, and are only able to discharge slowly. Nickel-iron batteries have long been used in European mining operations because of their ability to withstand vibrations, high temperatures and other physical stress. They are being examined again for use in wind and solar power systems and for modern electric vehicle applications. In many respects the Nickel/Iron battery was almost "too good". A battery that lasts for decades in many cases can outlast the equipment that it was originally designed to power. So from an economic standpoint lead acid, NiCd and other technologies have been deemed "good enough" and are the predominant technologies in use today even though they do not last as long as a Nickel/Iron counterpart. ElectrochemistryThe half reactions at anode and cathode are given by:
and
(Discharging is read left to right, charging is from right to left.) [2] HistorySwedish inventor Waldemar Jungner had invented the Nickel-Cadmium battery in 1899. Junger experimented with substituting iron for the cadmium in varying proportions, including 100% iron. Jungner had already discovered that the main advantage over the nickel-cadmium chemistry was cost, but due to the poorer efficiency of the charging reaction and more pronounced formation of hydrogen (gassing), the nickel-iron technology was wanting and was abandoned. Jungner never patented the iron version of his battery. The battery was developed by Thomas Edison in 1901, and used as the energy source for electric vehicles, such as the Detroit Electric. Edison claimed the nickel-iron design to be, "... far superior to batteries using lead plates and acid"(lead-acid battery). Jungner's work was largely unknown in the US until the 1940's when Nickel Cadmium batteries went into production there. A 50 Volt Nickel-Iron battery was the main power supply in the WWII German V2 Rocket (together with two 16 Volt accumulators which powered the four gyroscopes), with a smaller version used in the V1 flying bomb. (viz. 1946 Operation Backfire blueprints.) Edison's batteries were made from about 1903 to 1972 by the Edison Battery Storage Company located in East Orange, NJ. They were quite profitable for the company. In 1972 the battery company was sold to the Exide Battery Corporation which discontinued making the battery in 1975. Edison was disappointed that his battery was not adopted for starting internal combustion engines and that electric vehicles went out of production only a few years after his battery was introduced. He actually developed the battery to be the battery of choice for electric vehicles which he thought would be the preferred transportation mode in the early 1900's. The battery enjoyed wide use for railroad signaling, fork lift, and standby power applications. There are no Nickel Iron batteries manufactured in the Western world at this time (2007), but they are still manufactured in China. References
Categories: Nickel | Iron | Rechargeable batteries |
||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Nickel-iron_battery". A list of authors is available in Wikipedia. | ||||||||||||||||||||||