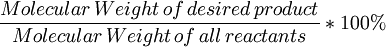To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Atom economyAtom economy describes the conversion efficiency of a chemical process in terms of all atoms involved. In an ideal chemical process the amount of starting materials or reactants equals the amount of all products generated (see stoichiometry) and no atom is wasted. Recent developments like high raw material (such as petrochemicals) costs and increased sensitivity to environmental concerns have made atom economical approaches more popular. Atom economy is an important concept of green chemistry philosophy. Product highlightAtom economy can be written as: % atom economy = Note that atom economy can be poor even when chemical yield is near 100%, see for instance the Cannizzaro reaction. A Diels-Alder reaction is an example of a potentially very atom efficient reaction.On the other hand if the desired product has an enantiomer the reaction needs to be sufficiently stereoselective even when atom economy is 100%. Atom economy can also be adjusted if a pendant group is recoverable, for example Evans auxiliary groups. However, if this can be avoided it is more desirable, as recovery processes will never be 100%. Atom economy can be improved upon by careful selection of starting materials and a catalyst system. Atom economy is just one way to evaluate a chemical process. Other criteria can include energy consumption, pollutants released and price. References
Categories: Stoichiometry | Green chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Atom_economy". A list of authors is available in Wikipedia. |