To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
AutocatalysisA single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product is itself the catalyst for that reaction. A set of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self sustaining given an input of energy and food molecules (see autocatalytic set).
Product highlight
Rate law in autocatalytic reactions
The rate law for the first order autocatalytical reaction The concentrations of A and B vary in time according to The graph for these equations is a sigmoid curve, which is typical for autocatalytical reactions: these chemical reactions proceed slowly at the start because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds a the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction is likely to be autocatalytic. Abiogenesis hypothesisBritish ethologist Richard Dawkins wrote about autocatalysis as a potential explanation for abiogenesis in his 2004 book The Ancestor's Tale. He cites experiments performed by Julius Rebek and his colleagues at the Scripps Research Institute in California in which they combined amino adenosine and pentafluorophenyl ester with the autocatalyst amino adenosine triacid ester (AATE). One system from the experiment contained variants of AATE which catalysed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection. Examples of autocatalytic reactions
Involvement in life processesTwo researchers, Robert Ulanowicz [1]and Stuart Kauffman, . [2] have suggested thatautocatalytic reactions played a central role in the evolution of life, and continue to constitute a basic element in life architecture. See also Relational order theories referencesCategories: Chemical processes | Chemical kinetics |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Autocatalysis". A list of authors is available in Wikipedia. |





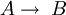 is
is ![\ v = k[A][B]](images/math/d/e/1/de10acc5c8a835e03ff527ffd5d6a61c.png) .
.
![[A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}}](images/math/7/0/2/702f04dd65537d65853d6785c376928d.png) and
and ![[B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}}](images/math/a/2/e/a2e8f292ed1676b68823e5e5bdcff432.png) .
.


