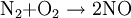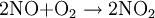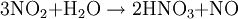To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Birkeland-Eyde processThe Birkeland-Eyde process was developed by the Norwegian industrialist and scientist Kristian Birkeland along with his business partner Sam Eyde. This process was used to fix atmospheric nitrogen which was in turn used to produce nitric acid, used for production of synthetic fertilizer. Factory based on the process was buildt in Rjukan and Notodden in Norway, combined with building of large hydropower facilities. The process is inefficient in terms of energy usage, and is today replaced by the Ostwald process, that produce nitrous acid from ammonia instead of air (usually from the Haber process). Product highlightThe processThis is a tedious process and the heating is carried out in the form of pulses by passing alternating current through an electric arc and suppressing the magnetic field created by it. Birkeland used a nearby hydroelectric power station for the electricity as this process demanded about 15000 KWH/Ton. The same reaction is carried out by lightning causing the temperature to rise to about 3000°C in the atmosphere to produce nitric oxide. The obtained nitric oxide is then oxidised to produce nitrogen dioxide. This nitrogen dioxide is then dissolved in water to give rise to nitric acid which is then purified by fractional distillation. Much purity is not obtained (68%) as nitric oxide and water form an azeotropic mixture |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Birkeland-Eyde_process". A list of authors is available in Wikipedia. |